Advanced Practice Nurse
Cardiology-Heart failure/Heart Transplant
Ann & Robert H. Lurie Children's Hospital
Serologic evaluation of pediatric heart transplant recipients for Hepatitis B
Grace Macek1, Kae Watanabe1,2, Taylor Heald-Sargent2,3.
1Pediatric Cardiology, Ann & Robert H Lurie Children's Hospital, Chicago, IL, United States; 2Pediatrics, Northwestern University, Chicago, IL, United States; 3Pediatric Infectious Diseases, Ann & Robert H Lurie Children's Hospital, Chicago, IL, United States
Introduction: Immunization before solid organ transplantation provides optimal prevention of post-transplant infectious diseases. The reason is two-fold as patients can be exposed to pathogens at the time of transplant from donor organs and patients may not respond as well after transplantation due to immunosuppression placing them at ongoing risk. Our institution has increasingly transplanted highly sensitized children. These patients undergo intense immunosuppression immediately peri-transplantation. We aimed to determine if our intensive immunosuppression depleted Hepatitis B antibodies and if patients listed for transplantation were able to seroconvert after immunization.
Methods: This is a retrospective study examining clinical characteristics, immunization status, and Hepatitis B serologies for patients that received a heart transplant at Ann & Robert H. Lurie Children’s Hospital between 6/2020 and 8/2021. Patients were excluded if they received prolonged or ongoing IVIG post-transplantation. Descriptive statistics were performed.
Results: A total of 25 patients were included. The median age at transplant was 11.4 years and 56% (n=14) were male. Reasons for transplant consisted of congenital heart disease in 52% (n =13), cardiomyopathy in 36% (n= 9) and re-transplantation in 12% (n=3). Nearly one-third of patients were considered sensitized pre-transplant (indicated by elevated panel reactive antibody (PRA)) and 36% (n=9) received plasmapheresis peri-operatively. B-cell depleting treatments included Rituximab for 7 patients (28%) and Bortezomib for 3 patients (12%). All patients received thymoglobulin for induction. Of the 22 that had both pre- and post- transplant serologies available, no patients that received plasmapheresis or B-cell depleting agents tested positive for Hepatitis B antibodies pre-transplant and then tested negative post-transplant. Fourteen patients were initially seronegative upon first testing. Most of the seronegative patients converted to seropositive (n= 8, 57%) with six having received additional doses of Hepatitis B vaccination per our medical records. Of the six patients that were seronegative throughout the study, four were given additional vaccinations without changing their antibody result.
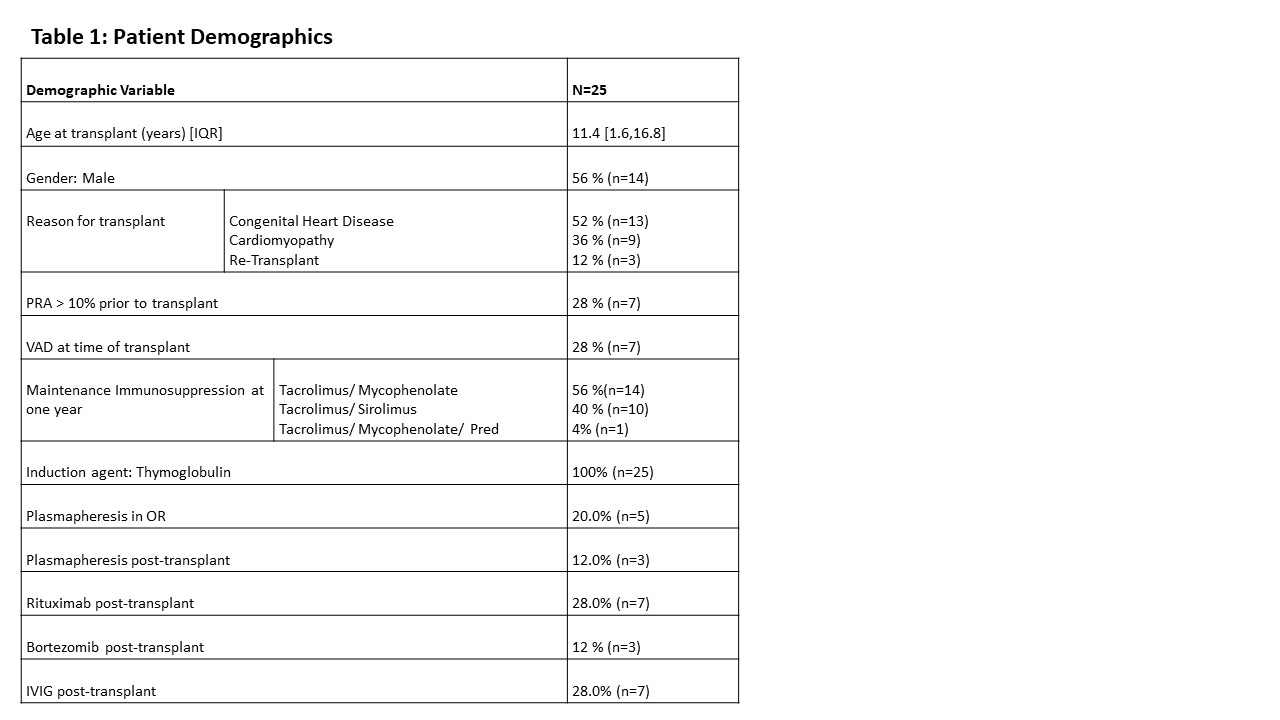
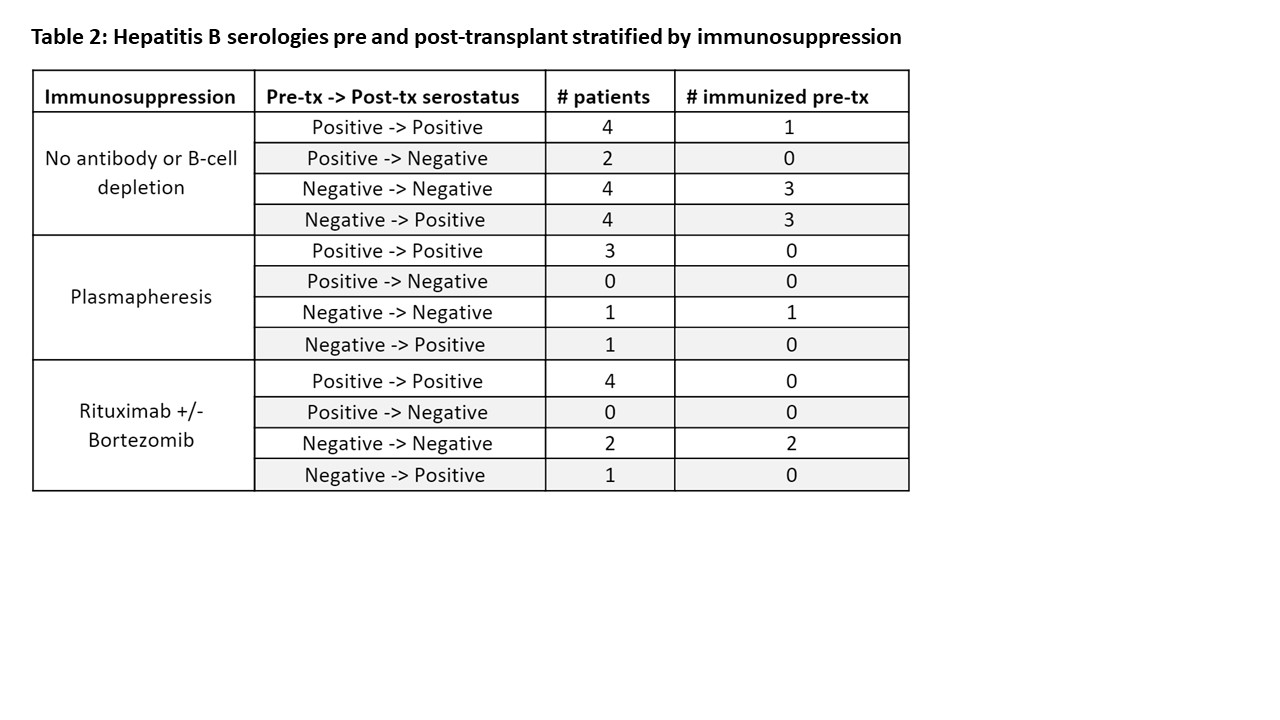
Conclusion: We found no relationship between a loss of antibody titers and immunosuppression regimen. The most important predictor of seroconversion was immunization. However, despite all patients having received at least one immunization prior to initial testing, fourteen were seronegative at initial evaluation. Immunization of these seronegative patients resulted in some seroconverting but not all responded to re-immunization. Interestingly, there were a small number of patients who seroconverted during the study period despite no documented immunization. Future studies with larger cohorts are needed to determine factors that may explain lack of serologic response to re-immunization.
Lectures by Grace Macek
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Tue-28 08:00 - 09:00 |
Infectious Diseases | Serologic evaluation of pediatric heart transplant recipients for Hepatitis B | Hill Country AB |
