Clinical characteristics and outcomes of COVID-19 in paediatric transplant recipients: an international retrospective study of the European reference network on transplantation in children (ERN-TransplantChild)
Luz Yadira Bravo Gallego1,2,3, Mara Cananzi4, Daniele Donà5, Elisa Benetti6, Marta González Vincent7, Esteban Frauca Remacha8,9, Paloma Jara Vega1,8,9.
1ERN-TransplantChild, La Paz University Hospital, Madrid, Spain; 2Lymphocyte Pathophysiology in Immunodeficiencies Group, Hospital La Paz Institute for Health Research (IdiPAZ), Madrid, Spain; 3CIBERER U767, Center for Biomedical Network Research on Rare Diseases, Madrid, Spain; 4Unit of Paediatric Gastroenterology, Digestive Endoscopy, Hepatology and Liver Transplantation, Women's and Children's Health, University Hospital of Padua, Padua, Italy; 5Pediatric Infectious Diseases Department, Women's and Children's Health, University Hospital of Padua, Padua, Italy; 6Pediatric Nephrology, Dialysis and Transplant Unit Department, Women's and Children's Health, University Hospital of Padua, Padua, Italy; 7Hematopoietic Stem Cell Transplantation Unit, Department of Pediatrics, Hospital Infantil Universitario Niño Jesús, Madrid, Spain; 8Pediatric Hepatology Department, La Paz University Hospital, Madrid, Spain; 9Molecular Hepatology Group, Hospital La Paz Institute for Health Research (IdiPAZ), Madrid, Spain
COVID-19 study group of the Clinical Audits Working Group - ERN-TransplantChild.
Introduction: The absence of robust data on COVID-19 prognosis in paediatric transplant recipients poses significant uncertainties regarding paediatric transplant activity and patient management. Moreover, it sustains patient and parental concerns regarding the risks related to SARS-CoV-2 infection per se and to chronic immunosuppressive therapy. The aim of the study was to characterize the clinical features, the outcome and survival rate of a large cohort of solid organ transplant (SOT) and haemopoietic stem cell transplant (HSCT) recipients.
Methods: A retrospective study was performed, with data collected between March 2020 to June 2021, among all 27 members and 15 member-candidates of the European Reference Network on Transplantation in Children (ERN-TransplantChild). The study was approved by the ethical committee of the ERN coordinator center (La Paz University Hospital, Madrid, Spain). Pediatric SOT and HSCT recipients (i.e., transplants performed <18 years of age) diagnosed with confirmed COVID-19 (according to the WHO 2020 definition) before 21 years of age were included in the study. Patient demographic and transplantation data, as well as COVID-19 clinical features and outcomes, were collected and statistically analyzed.
Results: Thirty-nine transplantation programs (34 SOT and 5 HSCT) from 11 European countries participated in the study. 120 confirmed COVID-19 cases (74 males) were reported in 109 SOT (4 heart, 31 kidney, 61 liver, 3 lung, 3 multivisceral, 7 combined) and 11 HSCT recipients (Fig. 1). The median age of the patients at the time of COVID-19 diagnosis and the median time from transplantation to COVID-19 diagnosis were respectively 132 months (IQR 72.5-188.5 months) and 48 months (IQR 16–108 months). 40% of children were asymptomatic. When present, fever (35.8%), respiratory (21.7%), and gastrointestinal (15%) symptoms were the most frequently reported and resolved within one week (78.5%). 86.7% of patients received no SARS-CoV-2-directed treatments and no adjustments of immunosuppressive treatment. Hospitalization related to SARS-CoV-2 infection occurred in 14% of the patients. Graft injury, mainly consisting of transient acute renal injury, was detected in 6.7% of children. Only two HSCT recipients with previous pulmonary morbidities required admission to PICU and died thereafter.
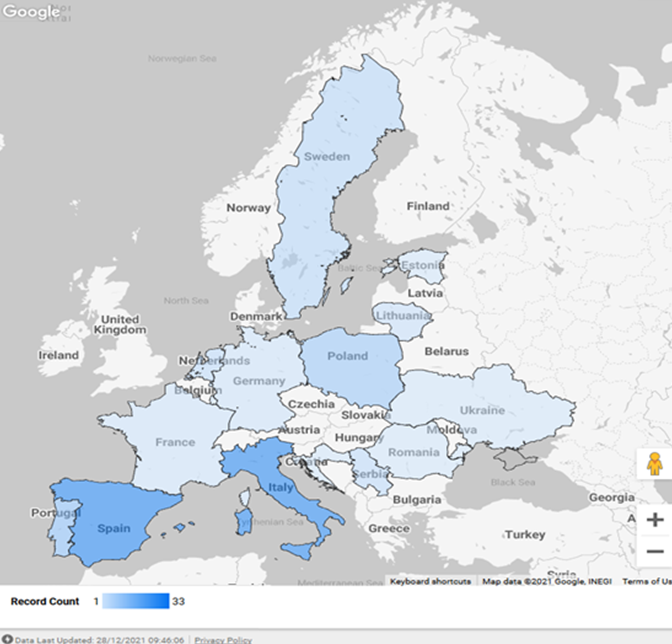
Conclusion: This study reports one of the largest series of COVID-19 in paediatric transplant recipients. While this population is theoretically more at risk for severe illness from SARS-CoV-2 infection due to ongoing immunosuppression and/or compromised immune system, our data show that, in this patient group, COVID-19 is mainly asymptomatic or mild, and seldom associated with patient death or graft loss.
This study has been supported by the ERN-TransplantChild, which is partly co-funded by the European Union within the framework of the EU4H Project Grants. Project number: 101085639..
Lectures by Ane Andres
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sun-26 10:00 - 11:00 |
Covid | Clinical characteristics and outcomes of COVID-19 in paediatric transplant recipients: an international retrospective study of the European reference network on transplantation in children (ERN-TransplantChild) | Hill Country AB |
|
Mon-27 10:00 - 11:00 |
Liver 2 | Impact and outcomes after optimization of liver bipartition in pediatric liver transplantation | Hill Country AB |
|
Tue-28 08:00 - 09:00 |
Surgery | First case report of multivisceral transplant from a deceased cardiac death donor | Hill Country CD |
