Director, Pediatric Transplantation
Pediatrics (Nephrology)
University of British Columbia / BC Children's Hospital
Bye Bye Biopsies? Evaluating clinical implementation of urinary CXCL10/creatinine monitoring in pediatric kidney transplant recipients
Ella Chan1, Jeffrey Bone2, Amy Thachil1,2, Kevin Vytlingam2, Tom Blydt-Hansen1,2.
1Department of Medicine , University of British Columbia, Vancouver, BC, Canada; 2BC Children's Hospital Research Institute, Vancouver, BC, Canada
ENMO.
Background: Urinary CXCL10 is a promising biomarker for kidney transplant rejection and also identifies other causes of allograft inflammation; however, studies evaluating approaches to effective clinical use and implementation are lacking.
Objectives: To re-validate thresholds for rejection diagnosis with urinary CXCL10/Creatinine (uCXCL10/Cr) monitoring. We then compared the efficacy of single vs two-step sequential test approaches to identify non-rejection causes for uCXCL10/Cr elevation; and need for biopsy to rule out subclinical rejection.
Methods: Pediatric kidney transplant recipients with serial banked urine samples were tested for uCXCL10/Cr. Biopsy-day urine samples were used to validate existing thresholds for rejection diagnosis (at >90% specificity). Repeated measures ANOVA was performed to compare the uCXCL10/Cr at different severities of rejection. Since biopsy is indicated if no alternative cause of high uCXCL10/Cr is identified, decision to biopsy was modeled with first-positive vs. 2-test persistent positive approach, which included screening for UTI, BKV, CMV, clinical rejection (high Cr). Two serial tests were included if separated by <4 months, and yield was defined as proportion without other diagnosis having subclinical rejection.
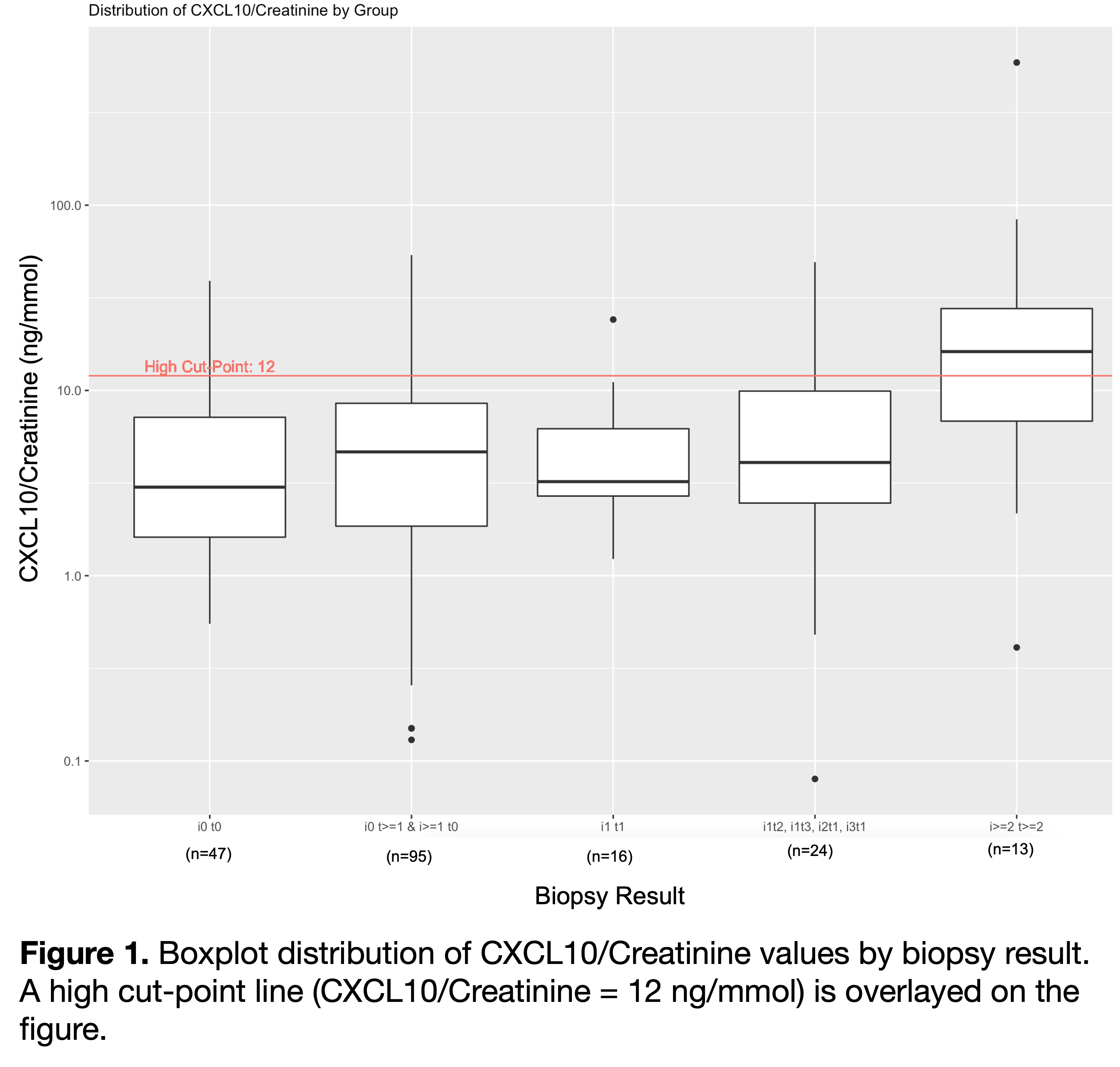
Results: Seventy patients aged 10.4±5.6 years at transplant, 61.3% male had n=720 samples analyzed. . Biopsy samples at 4.1±3.5 years post-transplant were grouped into no rejection (n=47), borderline (n=135), Banff 1A (n=13). ROC analysis validated the prior threshold of 12 ng/mmol (AUC = 0.75, 95% CI = 0.58-0.93). uCXCL10/Cr level increased progressively with Banff scores >i1t1 (p=0.021). Mean uCXCL10/Cr for Banff1A (2.7±0.8) was significantly greater than i0t0 (1.2±1.1; p=0.002). uCXCL10/Cr is relatively insensitive to rejection within the borderline grade.
A first-positive test approach (n=56) identified graft inflammation as CMV disease (n=4), clinical acute rejection (n=3), UTI (n=8), BK viremia (n=11), leaving 30 cases (54%) potentially in need of biopsy to rule out subclinical rejection. A two-step serial test approach further identified 16 cases with transient elevation, an additional case of clinical rejection (n=1), leaving only 14 (25%) without a clinical diagnosis (including n=5 without a second test). Of the 9 with no clinical diagnosis after 2-tests, n=5 had a biopsy with a yield of 4 (80%) with subclinical rejection. n=3 did not have a biopsy to exclude rejection.
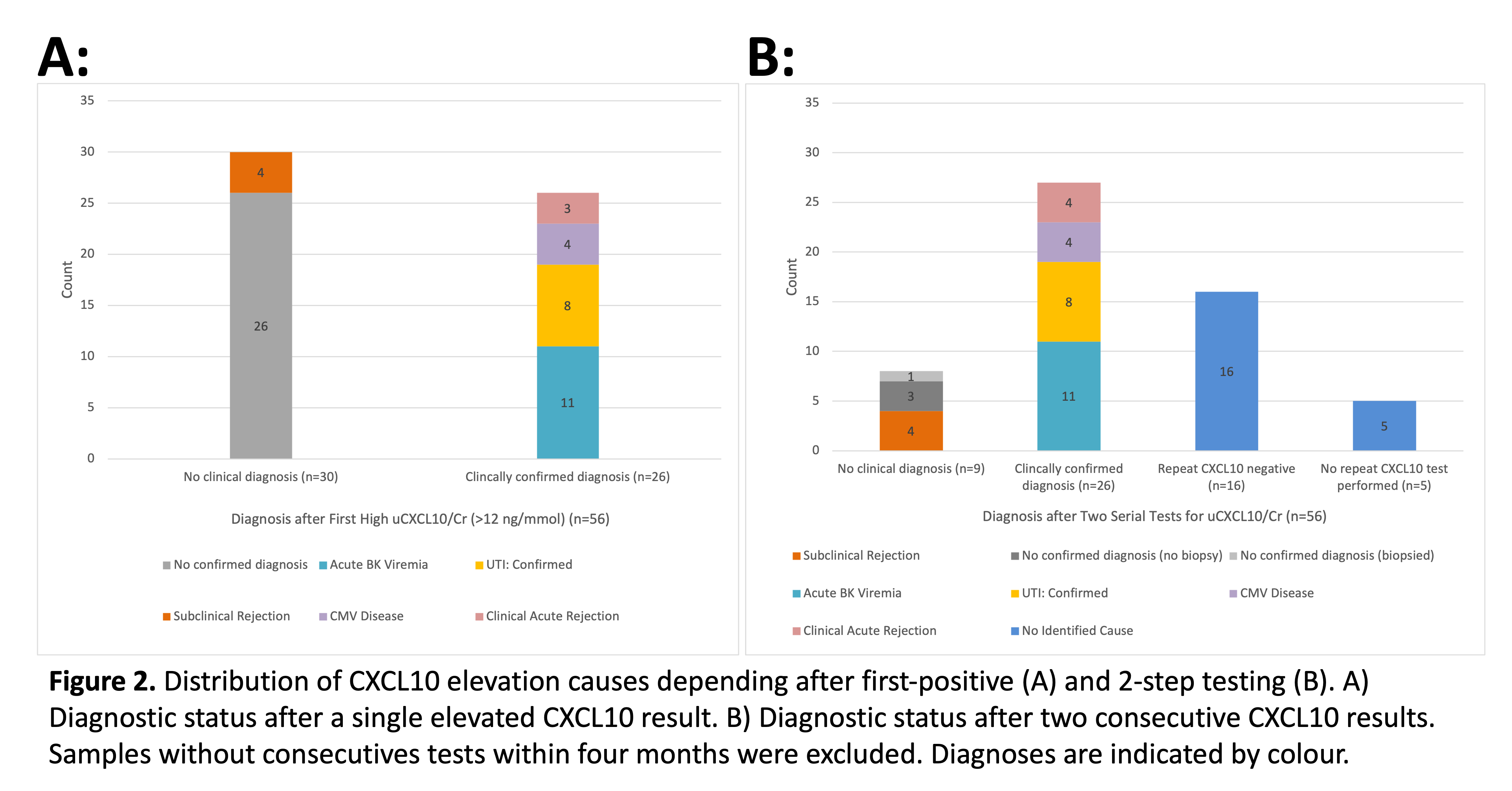
Significance: This work confirmed uCXCL10/Cr diagnostic thresholds to indicate biopsy for rejection risk (>90% specificity). A 2-step approach to diagnosis is superior to first-positive test for identifying alternate causes of graft inflammation, which improves yield for kidney biopsy to detect subclinical rejection.
Candice Wiedman. Monica Ho. BCCHR Summer Studentship Program.
Lectures by Tom Blydt-Hansen
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sun-26 10:00 - 11:00 |
Ethical/Psychosocial and Economical Issues | Exploring the ethical considerations of direct contact in pediatric organ transplantation: A qualitative study | Hill Country CD |
|
Sun-26 13:45 - 14:45 |
Kidney 2 | Bye Bye Biopsies? Evaluating clinical implementation of urinary CXCL10/creatinine monitoring in pediatric kidney transplant recipients | Zilker 3-4 |
