Vice Chairman
Department of Pediatrics I
University Children's Hospital Heidelberg
Emulation of the control cohort of a randomized controlled trial in pediatric kidney transplantation with Real-World Data from the CERTAIN Registry
Christian Patry1, Lukas D. Sauer2, Alexander Fichtner1, Luca Dello Strologo3, Dieter Haffner4, Jun Oh5, Ryszard Grenda6, Lars Pape7, Rezan Topaloğlu8, Lutz T Weber9, Antonia Bouts10, Agnieszka Prytula11, Jens König12, Britta Höcker1, Burkhard Tönshoff1.
1Department of Pediatric Nephrology, Zentrum für Kinder -und Jugendmedizin, Heidelberg, Heidelberg, Germany; 2Institute of Medical Biometry, University of Heidelberg, Heidelberg, Germany; 3Renal Transplant Unit, Bambino Gesù Children's Hospital, Pediatric subspecialities, Rome, Italy; 4Department of Pediatric Kidney, Liver and Metabolic Diseases, Hannover Medical School, Hannover, Germany; 5Pediatric Nephrology, University Hospital Hamburg, Hamburg, Germany; 6Department of Nephrology, Kidney Transplantation and Hypertension, Children's Memorial Health Institute, Warsaw, Poland; 7Clinic for Paediatrics III, Essen University Hospital, Essen, Germany; 8Department of Pediatric Nephrology, School of Medicine, Hacettepe University, Ankara, Turkey; 9Pediatric Nephrology, Children’s and Adolescents’ Hospital, University Hospital Cologne, Medical Faculty University of Cologne, Cologne, Germany; 10Department of Pediatric Nephrology, Amsterdam University Medical Center, Emma Children's Hospital, Amsterdam, Amsterdam, Netherlands; 11Pediatric Nephrology and Rheumatology Department, Ghent University Hospital, Ghent, Belgium; 12Department of General Pediatrics, University Children's Hospital, Münster, Münster, Germany
Background: Randomized controlled trials in pediatric kidney transplantation are hampered by low incidence and prevalence of kidney failure in children. Real-World Data from patient registries could facilitate the conduct of clinical trials by substituting a control cohort. However, the emulation of a control cohort by registry data in pediatric kidney transplantation has not been investigated so far.
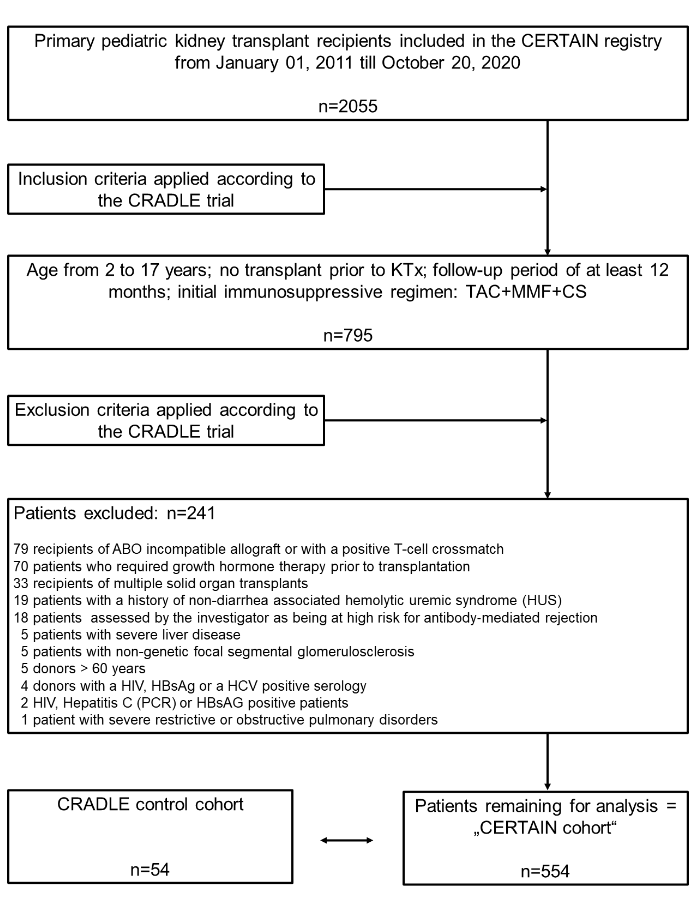
Methods: In this multicenter comparative analysis, we emulated the control cohort (n=54) of an RCT in pediatric kidney transplant patients (CRADLE trial; ClinicalTrials.gov NCT01544491) with data derived from the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN) registry, using the same inclusion and exclusion criteria (CERTAIN cohort, n=554). (Figure 1).
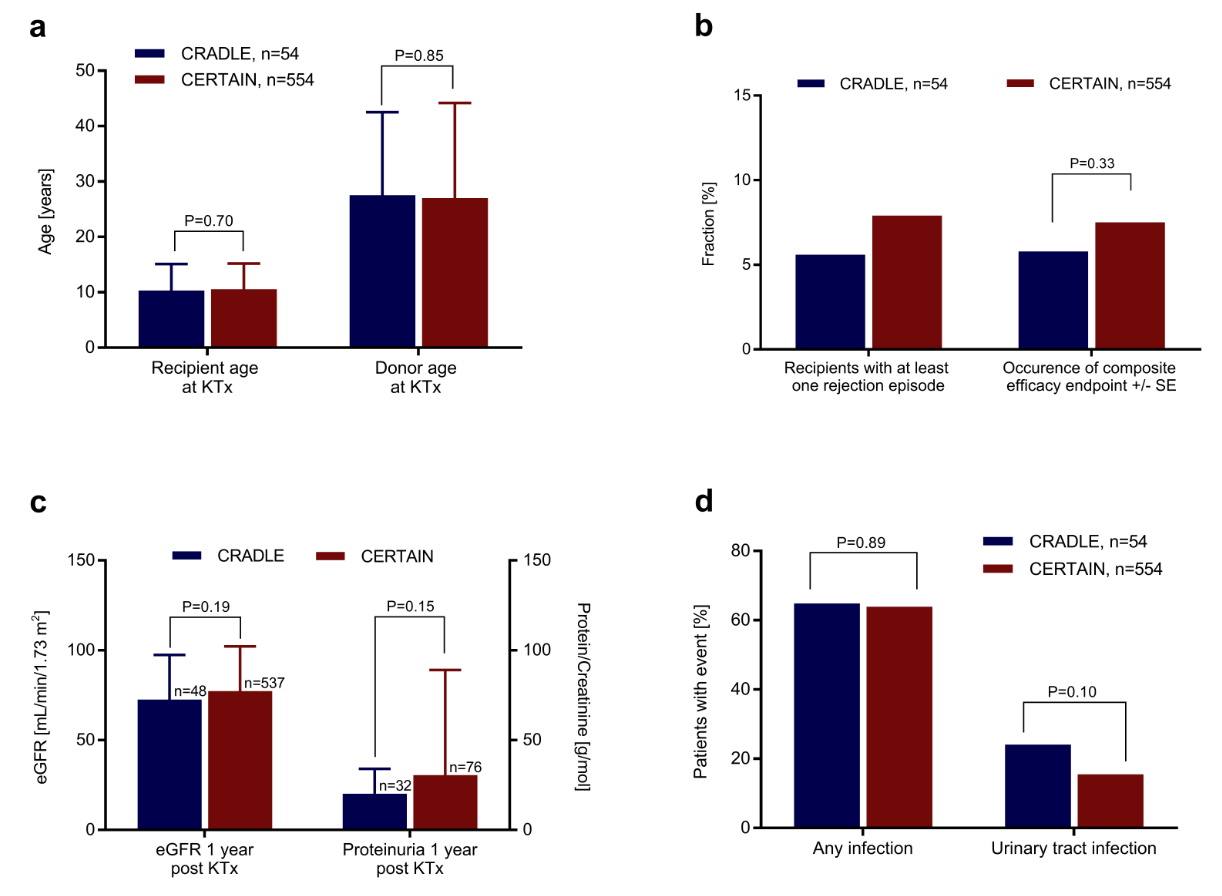
Results: Most baseline patient and transplant characteristics were well comparable between both cohorts. At year 1 posttransplant, a composite efficacy failure end point comprising biopsy-proven acute rejection, graft loss or death (5.8% ± 3.3% vs. 7.5% ± 1.1%, P=0.33) as well as renal function (72.5 ± 24.9 vs. 77.3 ± 24.2 mL/min/1.73 m2 P=0.19) did not differ significantly between CRADLE and CERTAIN. Further, the incidence and severity of BPAR (5.6% vs. 7.8%), the degree of proteinuria (20.2 ± 13.9 vs. 30.6 ± 58.4 g/mol, P=0.15) as well as key safety parameters such as occurrence of urinary tract infections (24.1% vs. 15.5%, P=0.10) were well comparable. (Figure 2).
Conclusions: In conclusion, usage of Real-World Data from patient registries such as CERTAIN to emulate the control cohort of an RCT is feasible and could facilitate the conduct of clinical trials in pediatric kidney transplantation.
