Utility of donor-derived cell-free DNA and tacrolimus variability in monitoring early post-kidney transplant outcomes
Rohit George1, Gina Aeckersberg2, Kelley Hitchman3, Elisabeth Kincaide4, Daniel Ranch2, Kim Piburn2.
1Department of Pediatrics, University of Texas Health San Antonio, San Antonio, TX, United States; 2Department of Pediatrics, Division of Pediatric Nephrology, University of Texas Health San Antonio, San Antonio, TX, United States; 3Department of Pathology and Laboratory Medicine, University of Texas Health San Antonio, San Antonio, TX, United States; 4Pharmacy Services, University Health Transplant Institute, San Antonio, TX, United States
Introduction: Donor-derived cell-free DNA (dd-cfDNA) has been proposed as a prognostic biomarker for diagnosis of acute rejection in kidney transplant recipients (1). Though elevation in cfDNA levels signifies tissue injury, cfDNA alone is not specific to acute rejection, as cfDNA levels can be elevated in other processes causing cellular injury (2). Studies have also shown that high tacrolimus variability has been associated with the development of de novo donor-specific antibodies (dnDSA) and graft loss. In this study, we investigated the combination of dd-cfDNA and tacrolimus variability in association with early de novo DSA formation in pediatric kidney transplant recipients.
Methods: Pediatric patients who underwent kidney-only transplantation at a single center from 2021-2022 were included in the study. Patients were followed until 9/23/2022 or until graft loss. Tacrolimus variability was defined using the coefficient of variation (CV; standard deviation/mean x 100%) of all tacrolimus levels obtained in the first two years post-transplant. Peak dd-cfDNA values were included in the analyses, and dd-cfDNA values were censored at time of dnDSA detection. Outcomes of interest were de novo DSA formation and graft loss. Survival curves were generated via the Kaplan-Meier method using the log-rank test for significance using Prism 9.
Results: A total of 29 patients were identified. Of those, 7 developed dnDSA. There were no cases of graft loss. Median tacrolimus CV was 45.4% (IQR 35.8-45.4). Median peak dd‑cfDNA value was 0.59% (IQR 0.36-1.025). Patients with tacrolimus variability >/= 30% and dd‑cfDNA >/= 0.5% had increased risk of dnDSA formation, p = 0.029 [Figure 1].
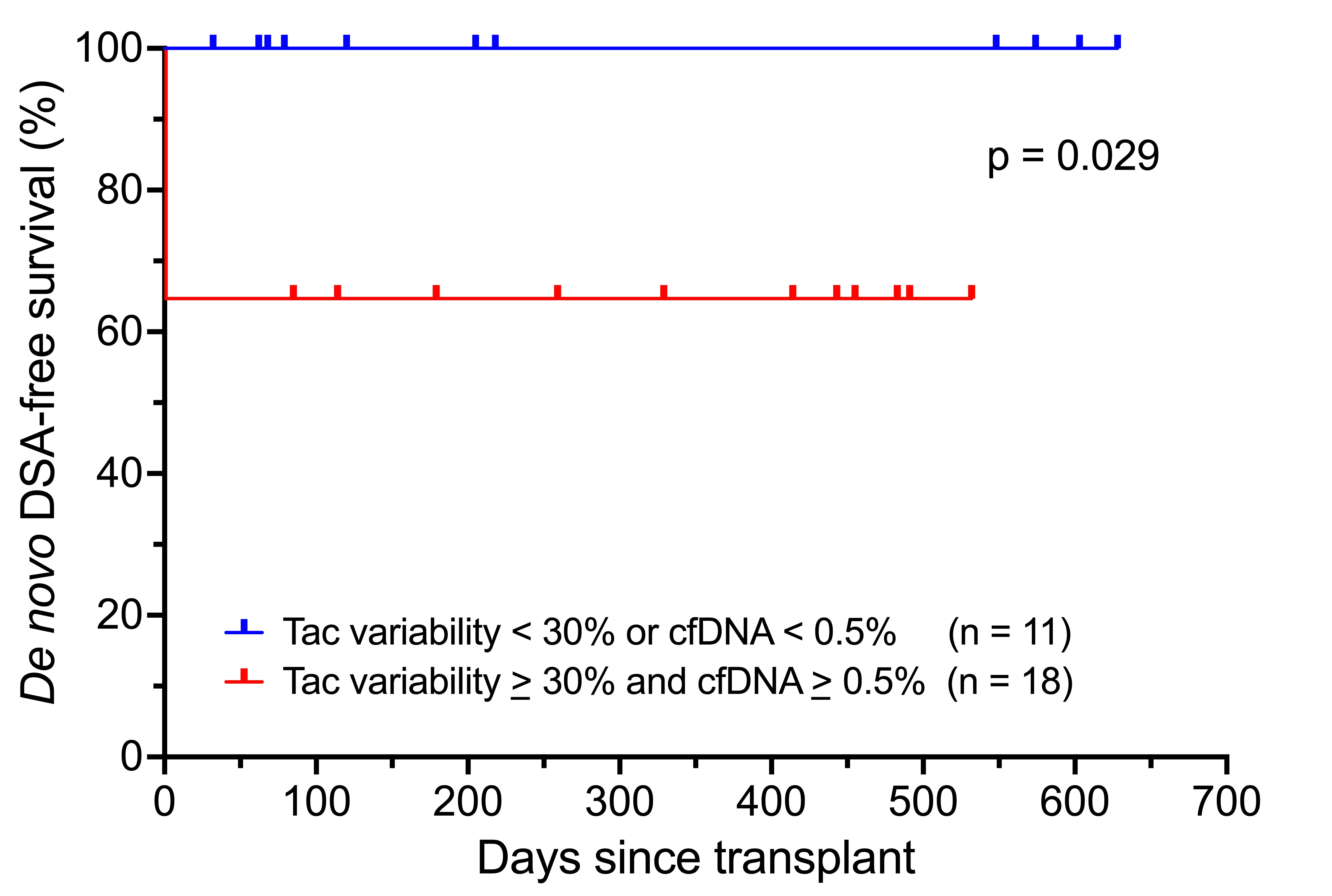
Conclusion: High donor-derived cell-free DNA in combination with high tacrolimus variability was associated with dnDSA formation. While dd-cfDNA is not specific for rejection, the addition of tacrolimus variability to dd-cfDNA to create a composite risk factor can help identify patients at increased risk for poor graft outcomes in the early post-transplant period.
References:
[1] Roy D. Bloom et al. Cell-Free DNA and Active Rejection in Kidney Allografts. JASN. Jul 2017, 28 (7) 2221-2232; DOI: 10.1681/ASN.2016091034.
[2] Thongprayoon C, Vaitla P, Craici IM, Leeaphorn N, Hansrivijit P, Salim SA, Bathini T, Rivera FHC, Cheungpasitporn W. The Use of Donor-Derived Cell-Free DNA for Assessment of Allograft Rejection and Injury Status. J Clin Med. 2020 May 14;9(5):1480. doi: 10.3390/jcm9051480. PMID: 32423115; PMCID: PMC7290747.
Lectures by Rohit George
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Mon-27 10:00 - 11:00 |
Kidney 3 | Utility of donor-derived cell-free DNA and tacrolimus variability in monitoring early post-kidney transplant outcomes | Zilker 3-4 |
