Outcomes of Basiliximab and Rapid Steroid Withdrawal versus Thymoglobulin Induction in Pediatric Kidney Transplant Recipients
Benjamin Steinman1, Stella Kilduff1, Nicole Hayde1.
1Pediatric Nephrology, Children's Hospital at Montefiore, Bronx, NY, United States
Introduction: Induction and maintenance immunosuppression protocols that incorporate steroid avoidance or withdrawal are increasingly common (1, 2). Few pediatric studies have evaluated Basiliximab with a rapid steroid withdrawal protocol (3, 4). We present a single center retrospective study comparing Basiliximab with rapid steroid withdrawal to Thymoglobulin induction and associated graft outcomes.
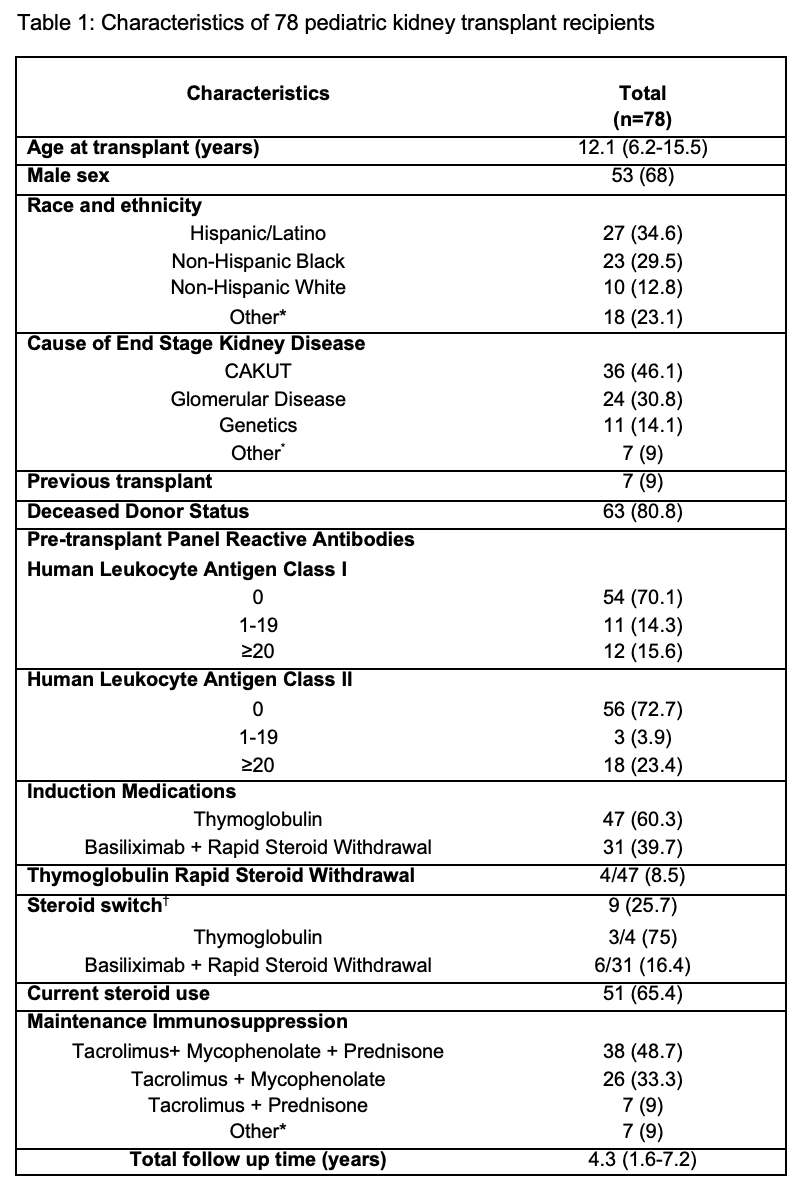
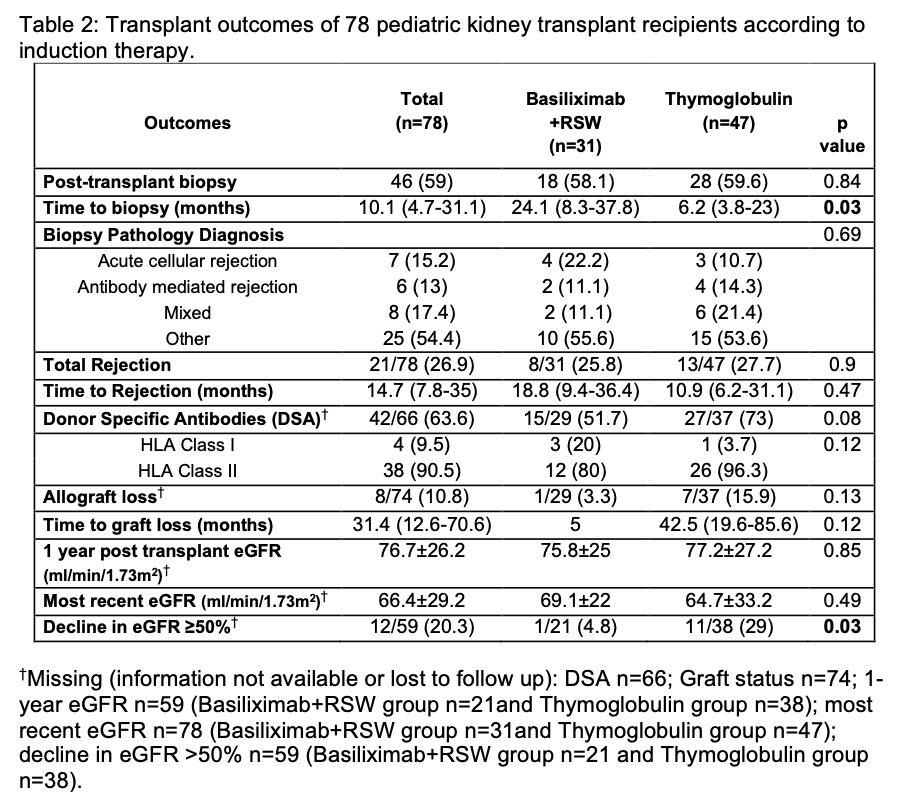
Methods: All patients who underwent kidney transplantation at the Children’s Hospital at Montefiore from August 2009-February 2022, were available for inclusion. A total of 78 patients were identified and followed post transplantation. Descriptive analysis of the study sample was performed comparing two induction therapy groups: Basiliximab + RSW and Thymoglobulin. Patients with pre-transplant PRA ≥20% or pre-formed donor specific antibodies (DSA) received thymoglobulin as per center transplant protocol. Steroid switch was defined as patients who underwent rapid steroid withdrawal post-transplant and later switched to steroid use. Analysis of transplant outcomes included time to biopsy, rejection, de novo DSA, eGFR at 1 year and most recent eGFR post-transplant, ≥50% decline in eGFR from 1-year post-transplant to most recent eGFR, and allograft loss.
Results: A total of 78 patients were identified and followed for a mean follow-up time of 4.7±3.6 years (Figure 1). Of the 78 patients, 31 (39.7%) received Basiliximab with RSW and 47 (60.3%) patients received Thymoglobulin induction. Four patients in the Thymoglobulin induction group underwent RSW. Among both groups with RSW, 9 patients underwent steroid switch (75% in Thymoglobulin group and 16.4% in the Basiliximab group). Patients in the thymoglobulin induction group had a significantly shorter time to clinically indicated biopsy compared to Basiliximab+RSW group (p=0.03) (Figure 2). A higher proportion of patients in the Thymoglobulin group developed de novo DSA when compared to the Basiliximab+RSW group, although this was not statistically significant (73 vs. 51.7%). In both groups there was an immunodominance of HLA Class II antibodies amongst those with DSA. There was no significant difference in time to rejection or frequency of rejection (Thymoglobulin 27.7% vs Basiliximab + RSW 25.8%), eGFR at 1-year post-transplant, most recent eGFR, and allograft loss between groups. However, 29% of patients who received Thymoglobulin had ≥50% decline in rate of eGFR compared to 4.8% in the Basiliximab+RSW group (p=0.03).
Conclusion: We conclude that the use of Basiliximab with rapid-steroid withdrawal may be safe in select pediatric kidney transplant recipients.
[1] Ekberg, J., Baid-Agrawal, S., Jespersen, B., Källén, R., Rafael, E., Skov, K., & Lindnér, P. (2021). A Randomized Controlled Trial on Safety of Steroid Avoidance in Immunologically Low-Risk Kidney Transplant Recipients. Kidney international reports, 7(2), 259–269
[2] Haller, M. C., Royuela, A., Nagler, E. V., Pascual, J., & Webster, A. C. (2016). Steroid avoidance or withdrawal for kidney transplant recipients. The Cochrane database of systematic reviews, 2016(8), CD005632
[3] Delucchi, A., Valenzuela, M., Ferrario, M., Lillo, A. M., Guerrero, J. L., Rodriguez, E., Cano, F., Cavada, G., Godoy, J., Rodriguez, J., Gonzalez, C. G., Buckel, E., & Contreras, L. (2007). Early steroid withdrawal in pediatric renal transplant on newer immunosuppressive drugs. Pediatric transplantation, 11(7), 743–748
[4] Nehus, E. J., Liu, C., Lu, B., Macaluso, M., & Kim, M. O. (2017). Graft survival of pediatric kidney transplant recipients selected for de novo steroid avoidance-a propensity score-matched study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association, 32(8), 1424–1431.