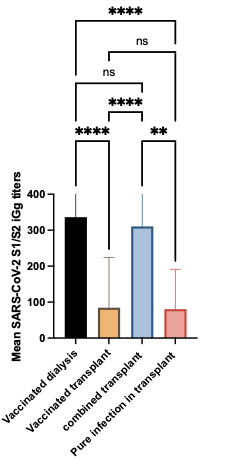Humoral immune response to SARS-CoV-2 BNT 162b2 vaccine in pediatric kidney transplant recipients and dialysis patients
Alanoud Alshami1, Hebatallah Bahbah1, Rabab RA Alattas1.
1Pediatric Nephrology and Kidney Transplant, King Fahad Specialist Hopsital-Dammam, Dammam, Saudi Arabia
Introduction: Since the start of the COVID-19 pandemic, data published on the immunogenicity of the SARS-CoV-2 BNT 162B2 vaccine in pediatric patients receiving renal replacement therapy is scanty.
The primary objective is to study this population's humoral immune response to the COVID-19 vaccine.
Methods: Pediatric kidney transplant (PKT) recipients and patients on dialysis who received two doses of the SARS-CoV-2 vaccine were included. Transplant and dialysis children who had PCR-positive COVID-19 infection during the study, regardless of their vaccine status, were also included. SARS- CoV-2 anti-spike protein (S1/S2) IgG was measured after the second dose of vaccine and after PCR-positive COVID-19 infection as routine clinical practice. Data on demographics, primary disease, induction, maintenance immunosuppressants, type of transplant, and post-transplant or dialysis duration were included.
Results: Of the 61 patients, 19 were dialysis, and 42 were kidney transplant recipients. All dialysis and 78.6% (33/42) transplant recipients received two doses of the SARS-CoV-2 BNT 162b vaccine. 21.4% (11/33) transplant recipients who received vaccination developed COVID-19 infection at a median time of 13 days post the second dose of vaccine. Nine transplant patients had pure COVID-19 infection without vaccination. The seroconversion rate in dialysis patients was 94.7% (18/19) compared to 50% (11/22) in transplant recipients (P< 0.001). Median S1/S2 IgG titers for dialysis patients were 337.2 Au/ml vs. 84.5 Au/ml in PKT (P< 0.0001). PKT with combined vaccine and infection had a higher titer than only vaccinated patients (310.3 vs. 84.5, p<0.0001). There were no clear predictors for seroconversion in PKT recipients.
Conclusion: Humoral immune response to SARS-CoV-2 BNT 162b2 vaccine in PKT recipients is suboptimal compared to dialysis patients and PKT recipients who received the vaccine and developed an infection