Devprakash Choudhary, India has been granted the TTS Scientific Congress Award

Graft loss due to isolated Angio-invasive Aspergillosis of Kidney Allograft following Normothermic Machine Perfusion of Neonatal En-bloc kidney after Uncontrolled Circulatory Determination of Death
Devprakash Choudhary1, Ashish Sharma1, Deepesh B Kenwar1, Sarbpreet Singh1, Abhinav Seth1, Ritambhra Nada2, Ranjana Walker Minz3, Harbir Singh Kohli 4, Sujata Wangkheimayum5, Shiva S Patil1.
1Department of Renal Transplant Surgery, PGIMER Chandigarh, Chandigarh, India; 2Department of Pathology, PGIMER Chandigarh, CHANDIGARH, India; 3Department of Immunopathology , PGIMER Chandigarh, CHANDIGARH, India; 4Department of Nephrology, PGIMER Chandigarh, CHANDIGARH, India; 5Department of Biochemistry, PGIMER Chandigarh, CHANDIGARH, India
Introduction: Global shortage of kidneys has led to an increased acceptance of kidneys from donors after cardiac death (DCD). The usage of uncontrolled DCD (uDCD) kidneys remains restricted due to a higher rate of delayed graft function (DGF) and primary non-function (PNF). Normothermic machine perfusion (NMP) has successfully utilised discarded DCD kidneys. Microbial contamination has been reported during organ preservation. However, data on the microbiological safety of NMP remains non-existent. The normothermic milieu with a blood-based nutrient-enriched perfusion solution provides optimal growth for microbes, further accentuated by an immunosuppressed state of recipients in the immediate post-transplant period.
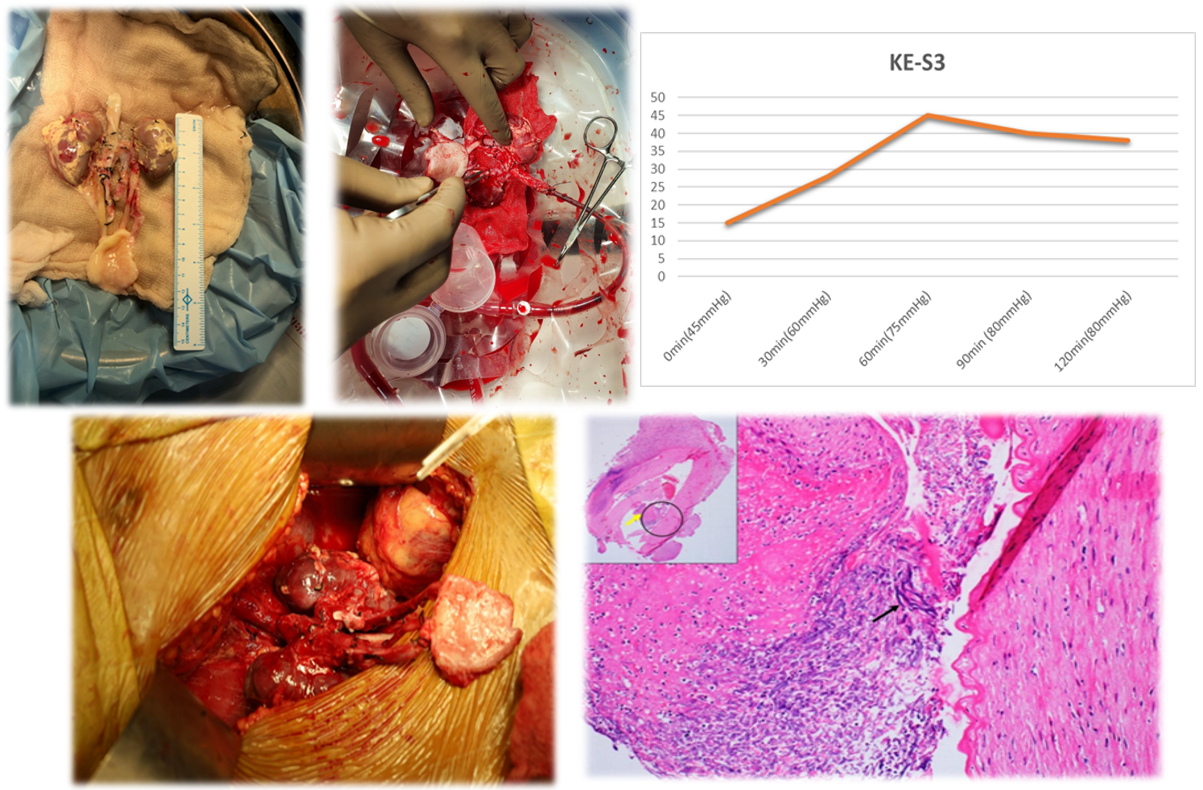
Methods: The indication for NMP was organ viability assessment in uDCD en-bloc kidneys. The neonatal donor kidneys underwent en-bloc NMP for two-hour on the Kidney AssistTM with an oxygenated red cell-based solution at 37°C in addition to static cold storage. Quality assessment score (QAS) and perfusion parameters on NMP were recorded. Post NMP, kidneys were flushed with histidine tryptophan ketoglutarate solution and underwent an en-bloc uDCD kidney transplantation.
Results: On NMP, neonatal en-bloc kidneys received a QAS score of 3 (1 for global pink appearance + 1 for Renal blood flow < 50mll/hr + 1 for urine output(U/O) <43 ml/hr) (table-1). The donor microbial screen and perfusion cultures were sterile. Graft doppler in the immediate post-op period revealed good intrarenal flow, and urine output improved to 300ml per day till POD 6 (Image-1). The recipient required exploration on day 7 for decreasing urine output with a peri-graft collection on graft ultrasonography, which turned out to be a urine leak from the protrusion of the DJ stent through graft parenchyma. Subsequently, U/O increased to 750 ml/day till day 30, when an open graft biopsy was performed for persisting graft dysfunction. On exploration, the kidneys showed patchy discolouration, and a graft nephrectomy was done. Biopsy showed acute T-cell-mediated rejection with Banff v3 lesions, acute cortical necrosis and invasive aspergillosis (Image -1). Antifungal therapy was given for six months, followed by a successful transplant after 15 months. To our knowledge, no fungal infection related to NMP has been reported in organs undergoing NMP.
Conclusions: NMP can potentially increase the organ donor pool. NMP microenvironment may act as an optimal medium for contaminated microbial overgrowth. The role of antimicrobials and optimal dosing on NMP is still indeterminate.
| Perfusion Parameters | 0min | 30min | 60min | 90min | 120min |
| pH | 6.88 | 6.92 | 6.96 | 7.6 | 7.54 |
| Na+(mmol/L) | 142 | 137.5 | 135 | 136 | 137 |
| K+(mmol/L) | 3.9 | 5.8 | 6.9 | 7.7 | 8 |
| pCo2(mmHg) | 14.1 | 12.5 | 15.2 | 8.6 | 13.7 |
| pO2(mmHg) | 471 | 542 | 556 | 489 | 532 |
| Lactate(mmol/L) | 3.46 | 4.8 | 4.95 | 5.4 | 6.45 |
| Ca++(mmol/L) | 0.42 | 0.3 | 0.29 | 0.2 | 0.2 |
| Glucose(mmol/dL) | 48 | 52 | 51 | 290 | 310 |
Received Funding from PGIMER Chandigarh.