Thrombotic microangiopathy after pediatric renal transplantation: report of 2 cases
Alejo de Sarasqueta1, Paula Bresso1, Oscar Amoreo 1, Demian Cursio1, Veronica Alejandra Bravo2.
1pediatric nephrology, Hospital El Cruce , Buenos Aires , Argentina; 2pediatric nephrology, Hospital Profesor Ramón Exeni , Buenos Aires , Argentina
Hospital El cruce -Buenos Aires Argentina.
Introduction: Post-renal transplant microangiopathy (TMA) is a considerable diagnostic challenge. Several factors may trigger it with or without genetic predisposition.
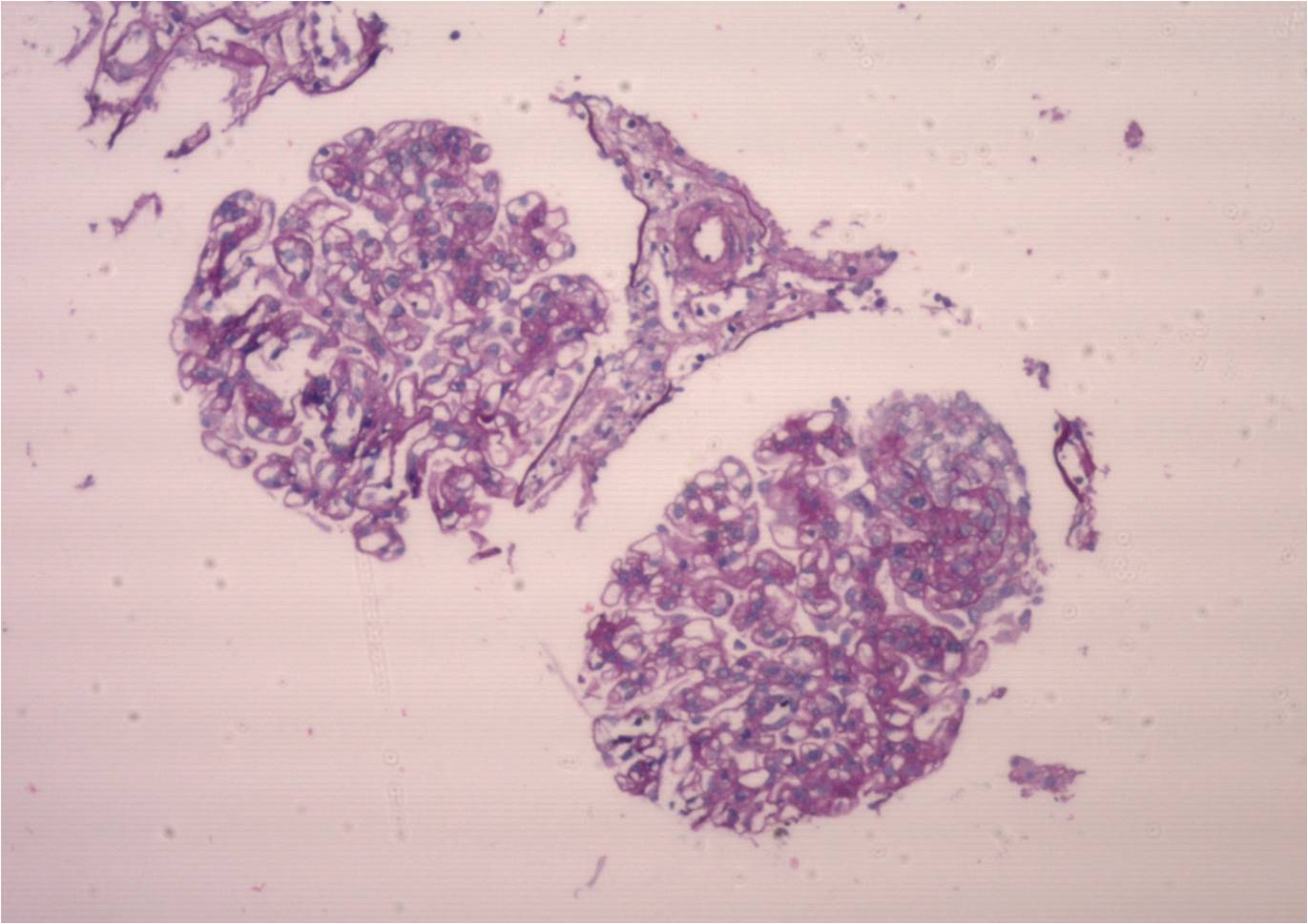
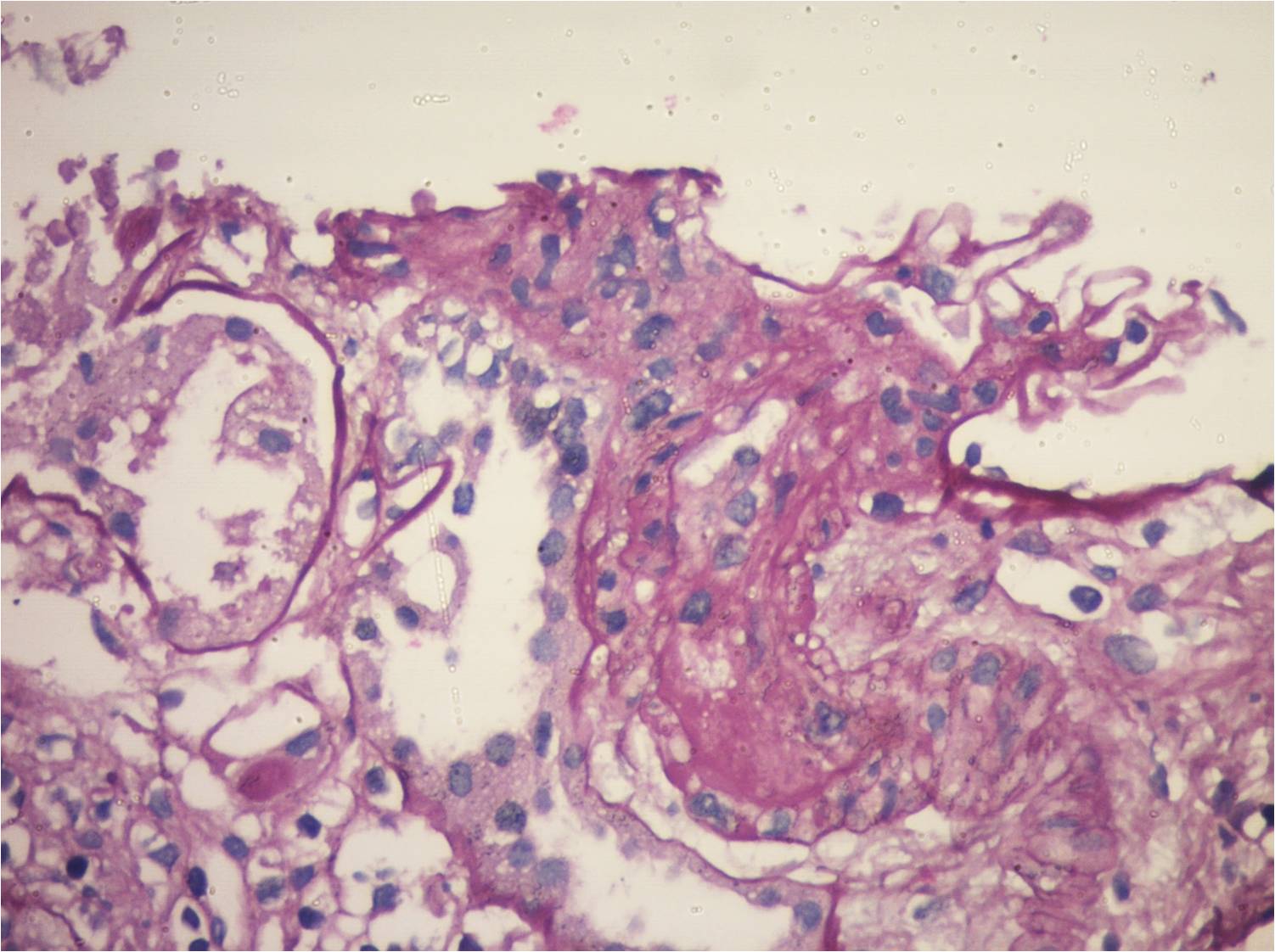
Case 1: 18-year-old male with stage 5 CKD caused by renal hypoplasia, when he received a deceased-donor kidney transplant (DDKT). He immediately developed severe hypotension, with initial graft dysfunction, undergoing transplactectomy 72 hours later due to renal vein thrombosis. Anti-HLA antibodies: negative; coagulation: normal. When he received a second DDKT (October 11, 2015), maintaining creatinine levels of 0.9-1 mg/dl, with negative proteinuria. On June 2, 2019 he went into hospital due to watery diarrhea; increased creatinine, anemia and low platelet counts; undetectable haptoglobin. He received pulses of methylprednisolone and plasmapheresis. Hemoglobin: 8.3 g/dl; Platelets: 75,000/mm3; LDH: 385; Haptoglobin: below 7; Coombs: negative; Creatinine: 2.3mg/dl; Tacrolimus dosage: 2.3 ng/ml;urine protein: 5 grams. Urine culture, blood culture x 2, serological tests and viral loads: negative; collagenogram: negative. Donor-specific anti-HLA antibodies: negative; renal biopsy: TMA; collapsing variant of focal segmental glomerulosclerosis; no signs of rejection; Treatment: tri-weekly plasmapheresis., the patient started receiving 900 mg of Eculizumab; C3 nephritic factor (NeF): negative;Factors B, H and I: normal; Anti-FH antibodies: undetectable; C5b-9 levels: elevated; aHUS clinical exome: no variants found. Stabilized kidney function and hematologic parameters.
Case 2: 17-year-old female. ESRD due to FSGS, starting at 9 years of age with RRT for 2 years when she received an unrelated living donor RT. At present, with chronic allograft nephropathy.Immunosuppression: Tacrolimus, Mycophenolate Sodium and Meprednisone.7 years after transplantation, she went into hospital due to abdominal pain and non-bloody diarrhea for 72 hours associated with oligoanuria. Evidence of microangiopathic hemolytic anemia (Hb: 8.4 gr/dl, schiszocytes +++, hematocrit 25%; platelets 100000, LDH 1110, Haptoglobin 5.8 mg/dl), (creatinine 8.1mg/dl, urea: 198 mg/dl); ;stool/urine cultures and fecal virological tests: negative; SARS-CoV-2 PCR negative; CMV, BKV and EBV PCR tests: negative; serological tests: negative. She started hemodialysis. Tacrolimus was shifted to Sirolimus; kidney biopsy:ongoing TMA, with global glomerulosclerosis of 60% and 80% IFTA. Anti-E. coli antibodies: IgM and IgG antibodies against LPS O145: POSITIVE. After 21 days of hospitalization, the patient was discharged with 3 mg/dl serum cr(GFR 29.5); preserved diuresis; proteinuria:500 mg/d; normal platelet count (365000) and hematocrit (31%); LDH: 193; FK dosage: 6.
Conclusion: Post-renal transplant TMS is multifactorial. With certain triggers, a group of predisposed patients will develop this complication. It is essential to establish the diagnostic and therapeutic criteria to optimize its management and prevent its recurrence.
Alta Complejidad .