LCP-Tacrolimus Extended-Release Tablets (Envarsus XR) Use in Pediatric Abdominal and Cardiac Solid Organ Transplant Recipients
Esther Bae1, Mary Chandran2, Melanie Everitt3, Margret Bock4.
1Pharmacy, Children's Hospital Colorado, Aurora, CO, United States; 2Pharmacy, UNC Hospitals and Clinics, Chapel Hill, NC, United States; 3Heart Institute, Children's Hospital Colorado, Aurora, CO, United States; 4Nephrology, Children's Hospital Colorado, Aurora, CO, United States
Purpose: Limited published experience describes once daily, extended-release tacrolimus (LCP-Tac) use in pediatric solid organ transplantation (SOT), particularly non-renal SOT. LCP-Tac can potentially simplify immunosuppression (IS) regimens, minimize immediate release-tacrolimus (IR-Tac)-associated adverse effects, and promote adherence. This study aims to evaluate safety of LCP-Tac use in adolescent and young adult (AYA) SOT populations.
Methods: This is a single-center, retrospective chart review of AYA SOT recipients (age < 22 years) converted from IR-Tac to LCP-Tac. Demographic and treatment data were collected up to 24 months following conversion. Graft survival and biopsy-proven acute rejection (BPAR) was assessed at five time points post-conversion (1, 3, 6, 12, and 24 months). Secondary outcome measures included infection rates, estimated glomerular filtration rate (eGFR), and effect on pill burden. Intra-patient variability of tacrolimus trough concentrations was assessed by the coefficient of variability (CV%) of serial tacrolimus trough concentrations prior to and post-conversion.
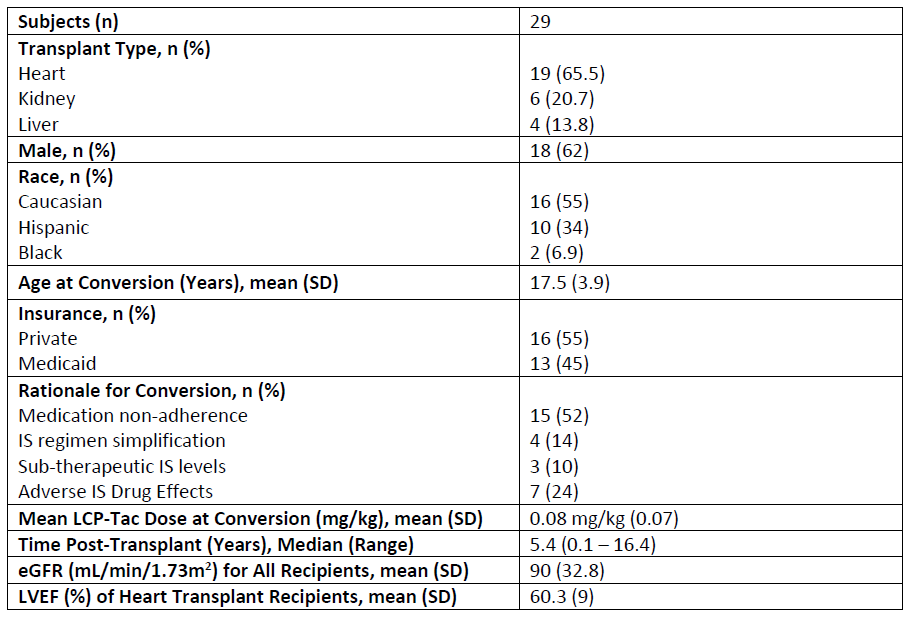
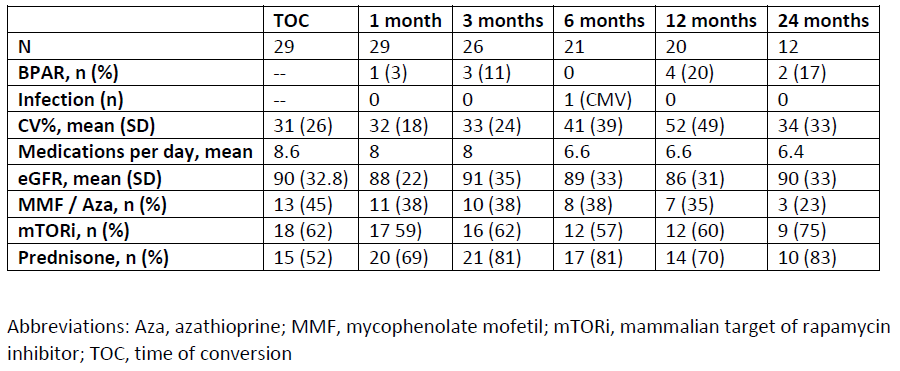
Results: Twenty-nine AYA SOT recipients (19 Heart, 6 Kidney, and 4 Liver) converted from IR-Tac to LCP-Tac. Mean age at conversion was 17.5 years, with youngest patient of 9 years. Median timing of conversion was 5.4 years post-transplant; however, 8 patients converted within the first-year. Conversion was most common for perceived or identified medication non-adherence. No graft loss occurred following conversion, and the BPAR incidence rate was similar to those reported for these populations. Infection occurred in only 1 patient (CMV viremia at 6 months post-conversion that resolved by 12 months). Renal function remained stable post-conversion over the 24-month period.
Conclusions: Conversion from IR-Tac to LCP-Tac is safe in a cohort of AYA SOT recipients. Though medication non-adherence was a primary reason for use of LCP-Tac, this study was not designed to evaluate impact of conversion on adherence. Pill burden was reduced, and CV% initially improved following conversion. Further research is needed to evaluate targeted use of LCP-Tac for IS tolerability and adherence in young SOT populations.
[1] Liverman R, Chandran MM, Crowther B. Considerations and Controversies of Pharmacologic Management of the Pediatric Kidney Transplant Recipient. Pharmacotherapy. 2020 Nov 5. https://doi.org/10.1002/phar.2483
[2] Moss M, Goebel J, Lofton A, Steinberg E, Bock M. Impact of Once-Daily Tacrolimus on Trough Concentration Variability in Stable Adolescent and Young Adult Renal Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). DOI: 10.1111/ajt.15405
[3] Budde, K., Bunnapradist, S., Grinyo, J.M., Ciechanowski, K., Denny, J.E., Silva, H.T., Rostaing, L. and (2014), Novel Once‐Daily Extended‐Release Tacrolimus (LCPT) Versus Twice‐Daily Tacrolimus in De Novo Kidney Transplants: One‐Year Results of Phase III, Double‐Blind, Randomized Trial. American Journal of Transplantation, 14: 2796-2806. https://doi.org/10.1111/ajt.12955
[4] Saengram, W., Vadcharavivad, S., Poolsup, N. et al. Extended release versus immediate release tacrolimus in kidney transplant recipients: a systematic review and meta-analysis. Eur J Clin Pharmacol 74, 1249–1260 (2018). https://doi.org/10.1007/s00228-018-2512-7
[5] Bunnapradist, S., Ciechanowski, K., West‐Thielke, P., Mulgaonkar, S., Rostaing, L., Vasudev, B., Budde, K. and (2013), Conversion From Twice‐Daily Tacrolimus to Once‐Daily Extended Release Tacrolimus (LCPT): The Phase III Randomized MELT Trial. American Journal of Transplantation, 13: 760-769. https://doi.org/10.1111/ajt.12035
[6] von Einsiedel J, Thölking G, Wilms C, Vorona E, Bokemeyer A, Schmidt HH, Kabar I, Hüsing-Kabar A. Conversion from Standard-Release Tacrolimus to MeltDose® Tacrolimus (LCPT) Improves Renal Function after Liver Transplantation. Journal of Clinical Medicine. 2020; 9(6):1654. https://doi.org/10.3390/jcm9061654
[7] Kim M, Givertz MM, Stewart GC, Page DS, Woodcome EL, Mehra MR. Efficacy and Safety of LCP-Tacrolimus (Envarsus XR®) Conversion Therapy in Heart Transplantation. J Heart Lung Transplant. 2020; 39(4): S502. https://doi.org/10.1016/j.healun.2020.01.102
[8] Uyanik-Uenal K, Moayedifar R, Osorio E, Aliabadi-Zuckermann A, Haberl T, Laufer G, Zuckermann A. First Experience with MeltDose-Tacrolimus (Envarsus) in Heart Transplantation. J Heart Lung Transplant. 2017; 36(4): S298-S299. https://doi.org/10.1016/j.healun.2017.01.1470
[9] Vila-Santandreu A, Calzada Y, Codina E. Single Centre Experience with the use of Envarsus in Pediatric Kidney Transplant Population. Transplantation, 2018; 102(7): S850.
[10] Patel, N., Cook, A., Greenhalgh, E., Rech, M. A., Rusinak, J., & Heinrich, L. (2016). Overview of extended release tacrolimus in solid organ transplantation. World journal of transplantation, 6(1), 144–154. https://doi.org/10.5500/wjt.v6.i1.144