Richard W. Issitt, United Kingdom has been granted the IPTA Scientific Congress Award

Richard Issitt gained his Doctorate in cardiopulmonary bypass related neurological injury from the University of Southampton. He is currently a Postdoctoral Clinical Research Fellow and Senior Paediatric Perfusionist at Great Ormond Street Hospital for Children NHS Foundation Trust (GOSH). Since 2018, he has also been Communications and Engagement Lead of the Digital Research Environment (DRE) for GOSH DRIVE, Great Ormond Street's Digital Research, Informatics and Virtual Environments Unit. Richard is also an Honorary Clinical Lecturer at the Centre for Heart Failure, Transplantation and Extracorporeal Support, part of University College London's Institute of Cardiovascular Science. Richard's research goal is to develop a bioinformatician-perfusionist role in the future to better understand how real-time intraoperative changes can influence long-term outcomes in children with cardiovascular diseases.
Dr Issitt has authored more than 60 research papers, and been cited appoximately 300 times. He has presented at international conferences around the globe. He teaches the paediatric module of the Bristol University M.Sc in Perfusion Science and is a reviewer for multiple peer review journals, as well as sitting on the Editorial Board of the journal Perfusion.
Dr Issitt is the only dedicated paediatric perfusionist in the UK with a Doctorate, and has taken the GOSH perfusion department from no publications 10 years ago, to over 30, establishing GOSH as the most active perfusion research department in the UK. He is one of only 3 perfusionists to win the Society of Perfusionist’s research award twice, and was the youngest perfusionist ever to be granted Fellowship to the College of Clinical Perfusionists in 2008.
Richard's research is funded by a British Heart Foundation Clinical Research Training Fellowship.
Use of immunoadsorption in an ex vivo laboratory setting to provide a potential intraoperative desensitisation methodology for paediatric cardiothoracic transplantation
Richard Issitt1,5,7, Eamonn Cudworth6, Mario Cortina Borja8, Arun Gupta6, Delordson M. Kallon6, Richard Crook1, Michael Shaw1, Alex Robertson1, Victor T. Tsang2,7, Sophie Henwood3, Vivek Muthurangu7, Neil J. Sebire5, Michael Burch3,4,7, Matthew Fenton3,4,7.
1Perfusion Department, Great Ormond Street Hospital for Children, Great Ormond Street Hospital, Great Ormond Street, United Kingdom; 2Cardiothoracic Surgery, Great Ormond Street Hospital for Children, Great Ormond Street Hospital, Great Ormond Street, United Kingdom; 3Cardiothoracic Transplantation, Great Ormond Street Hospital for Children, Great Ormond Street Hospital, Great Ormond Street, United Kingdom; 4Cardiology, Great Ormond Street Hospital for Children, Great Ormond Street Hospital, Great Ormond Street, United Kingdom; 5Digital Research Informatics and Virtual Environment, Great Ormond Street Hospital for Children, Great Ormond Street Hospital, Great Ormond Street, United Kingdom; 6Clinical Transplantation, Barts Health NHS Trust, London, United Kingdom; 7Institute of Cardiovascular Science, UCL, London, United Kingdom; 8Institute of Child Health, UCL, London, United Kingdom
Background: Anti-human leukocyte antigen (HLA) antibody sensitisation represents a major barrier to cardiac transplantation. Patients with heart failure, supported by Ventricular Assist Devices (VAD), or those who have undergone surgical repair for congenital heart defects are at higher risk of developing anti-HLA antibodies, and thus limiting their compatibility with potential donors. Currently there is no consensus as to how, or when desensitisation should take place, and through what mechanism, meaning that sensitised patient must wait for a compatible donor for many months, if not years. For many this delay in finding a suitable organ results in increased risk of complications from VADs, with poorer waiting-list outcomes. We aimed to determine if it were possible to use immunoadsorption in an ex vivo laboratory setting to provide a potential, intraoperative desensitisation methodology.
Methods: Anti-HLA antibody-containing whole blood was obtained from NHS Blood and Transplant and quantified using a Luminex Single Antigen Bead assay. A Cardiopulmonary bypass (CPB) circuit was set up to mimic a 20kg patient undergoing cardiac transplantation, into which a plasma separator was placed. Anti-HLA antibody containing plasma was then diverted to a standalone, secondary immunoadsorption system, with antibody-depleted plasma return to the CPB circuit.
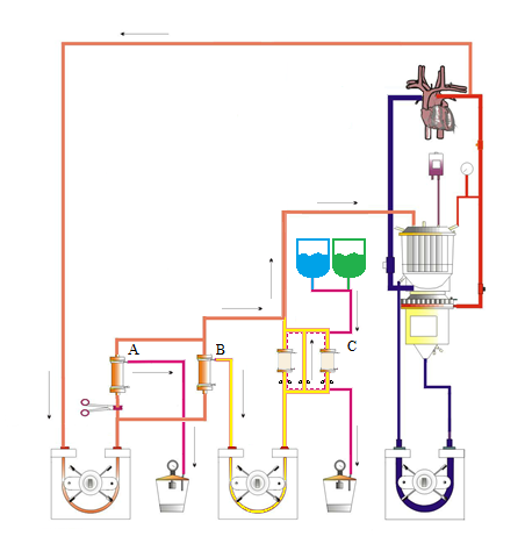
A total treatment volume of 3-fold the pseudo-patient’s plasma volume (4500ml at 75ml/kg) was treated. Samples for anti-HLA antibody quantification were taking at baseline, when combined with the CPB prime (on bypass), and then every 20 minutes for the duration of treatment (total 3 hours).
Results: All 3 experiments demonstrated a reduction in individual bead mean fluorescence intensity (MFI) to below clinically relevant levels (<1000 MFI), and in the majority of cases below the lower detection limit (<500 MFI), even in HLA specificities with a baseline MFI >4000. Reduction occurred in all cases within 120 minutes, demonstrating efficacy in a time period usual for cardiac transplantation.
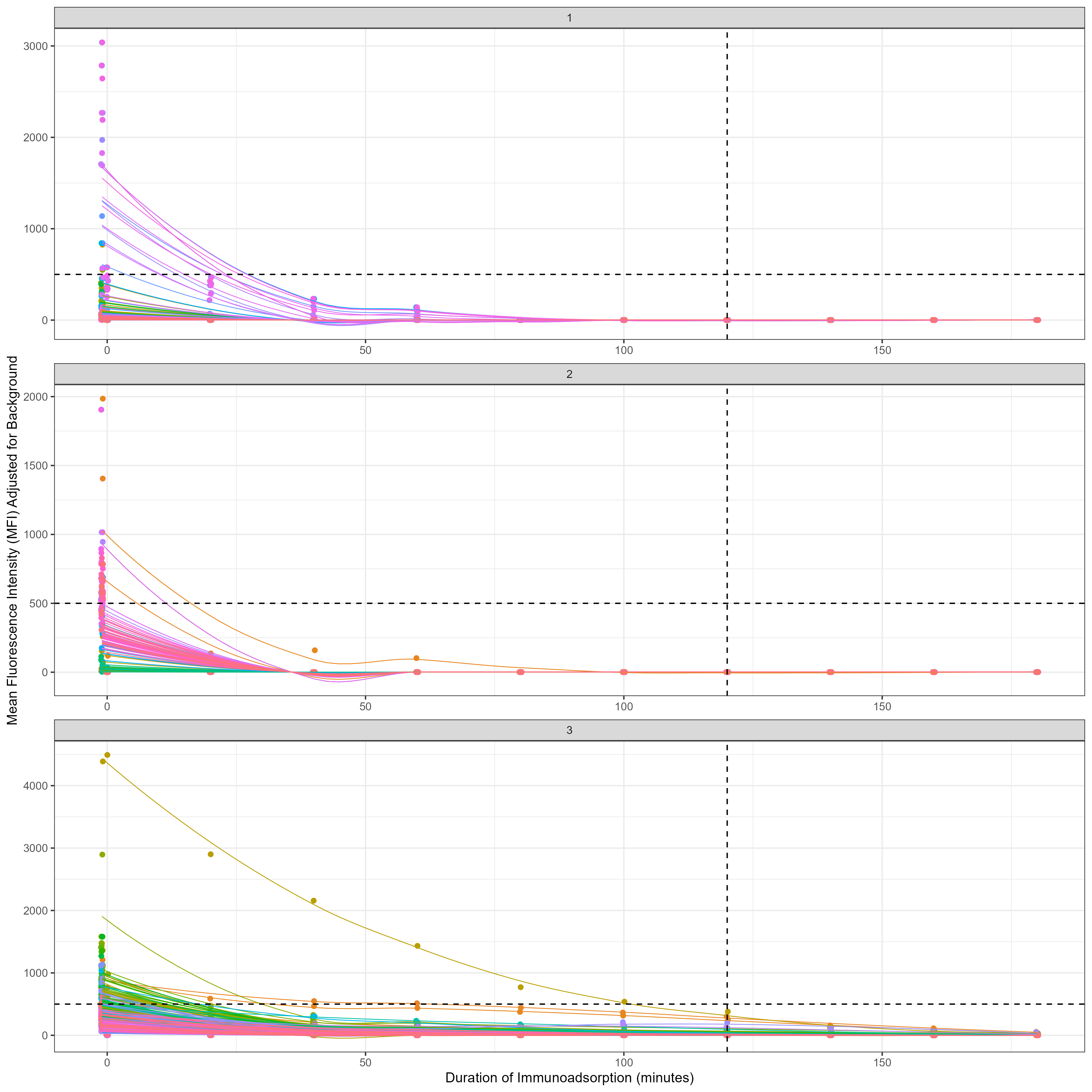
Flow cytometric crossmatching of suitable pseudo-patients demonstrated a flipping from T cell and B cell positive channel shifts to negative, demonstrating a reduction in binding capacity.
Conclusions: Intraoperative immunoadsorption in an ex vivo setting demonstrates a clinically relevant reduction in anti-HLA antibodies within the normal timeframe for cardiac transplantation. This method represents a potential desensitisation technique that could enable sensitised children to accept a donor organ earlier, even in the presence of donor-specific anti-HLA antibodies.
British Heart Foundation Research Fellowship to Richard Issitt (FS/19/52/34563).