
Bye Bye Biopsies? Evaluating clinical implementation of urinary CXCL10/creatinine monitoring in pediatric kidney transplant recipients
Ella Chan1, Jeffrey Bone2, Amy Thachil1,2, Kevin Vytlingam2, Tom Blydt-Hansen1,2.
1Department of Medicine , University of British Columbia, Vancouver, BC, Canada; 2BC Children's Hospital Research Institute, Vancouver, BC, Canada
ENMO.
Background: Urinary CXCL10 is a promising biomarker for kidney transplant rejection and also identifies other causes of allograft inflammation; however, studies evaluating approaches to effective clinical use and implementation are lacking.
Objectives: To re-validate thresholds for rejection diagnosis with urinary CXCL10/Creatinine (uCXCL10/Cr) monitoring. We then compared the efficacy of single vs two-step sequential test approaches to identify non-rejection causes for uCXCL10/Cr elevation; and need for biopsy to rule out subclinical rejection.
Methods: Pediatric kidney transplant recipients with serial banked urine samples were tested for uCXCL10/Cr. Biopsy-day urine samples were used to validate existing thresholds for rejection diagnosis (at >90% specificity). Repeated measures ANOVA was performed to compare the uCXCL10/Cr at different severities of rejection. Since biopsy is indicated if no alternative cause of high uCXCL10/Cr is identified, decision to biopsy was modeled with first-positive vs. 2-test persistent positive approach, which included screening for UTI, BKV, CMV, clinical rejection (high Cr). Two serial tests were included if separated by <4 months, and yield was defined as proportion without other diagnosis having subclinical rejection.
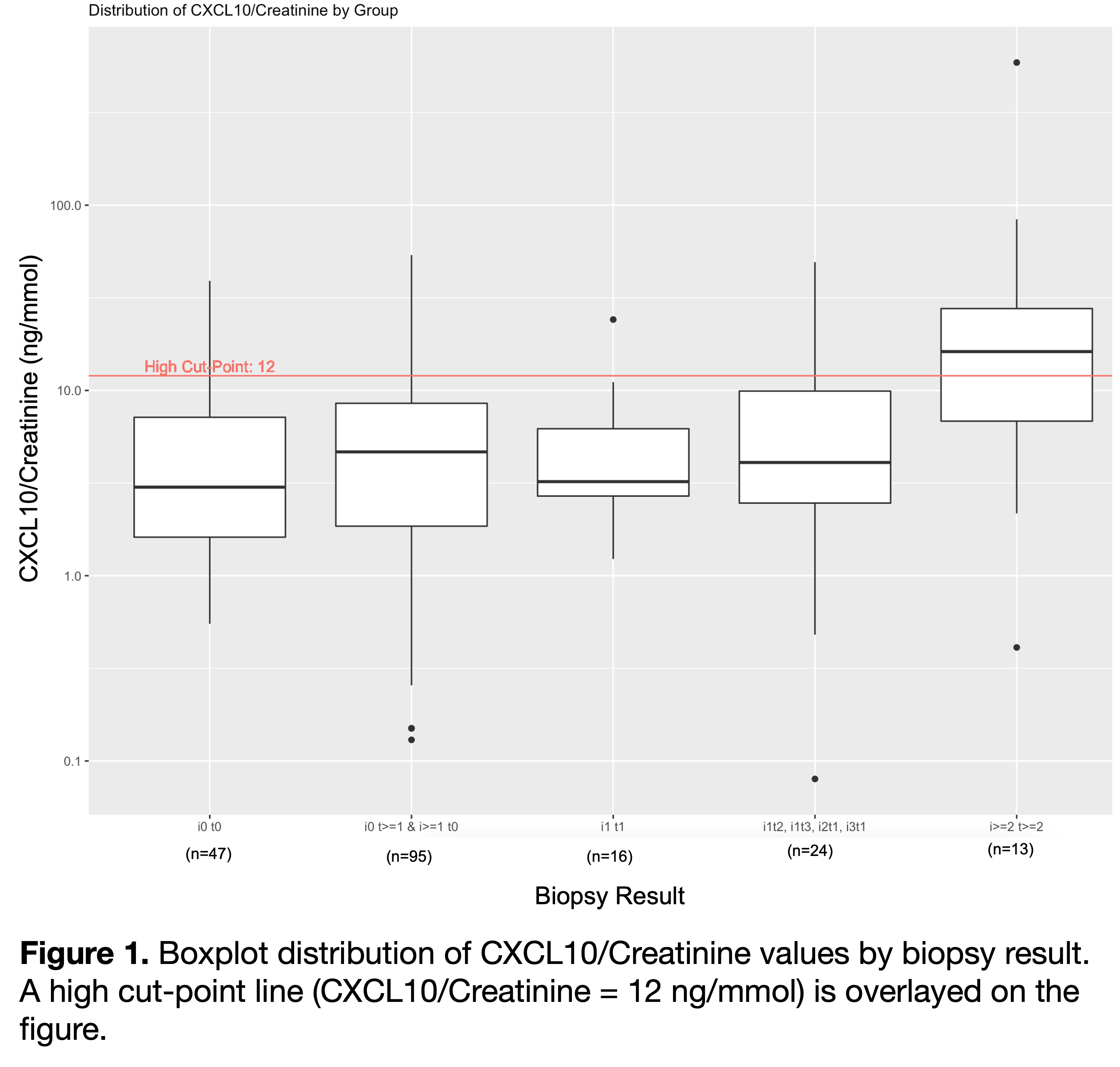
Results: Seventy patients aged 10.4±5.6 years at transplant, 61.3% male had n=720 samples analyzed. . Biopsy samples at 4.1±3.5 years post-transplant were grouped into no rejection (n=47), borderline (n=135), Banff 1A (n=13). ROC analysis validated the prior threshold of 12 ng/mmol (AUC = 0.75, 95% CI = 0.58-0.93). uCXCL10/Cr level increased progressively with Banff scores >i1t1 (p=0.021). Mean uCXCL10/Cr for Banff1A (2.7±0.8) was significantly greater than i0t0 (1.2±1.1; p=0.002). uCXCL10/Cr is relatively insensitive to rejection within the borderline grade.
A first-positive test approach (n=56) identified graft inflammation as CMV disease (n=4), clinical acute rejection (n=3), UTI (n=8), BK viremia (n=11), leaving 30 cases (54%) potentially in need of biopsy to rule out subclinical rejection. A two-step serial test approach further identified 16 cases with transient elevation, an additional case of clinical rejection (n=1), leaving only 14 (25%) without a clinical diagnosis (including n=5 without a second test). Of the 9 with no clinical diagnosis after 2-tests, n=5 had a biopsy with a yield of 4 (80%) with subclinical rejection. n=3 did not have a biopsy to exclude rejection.
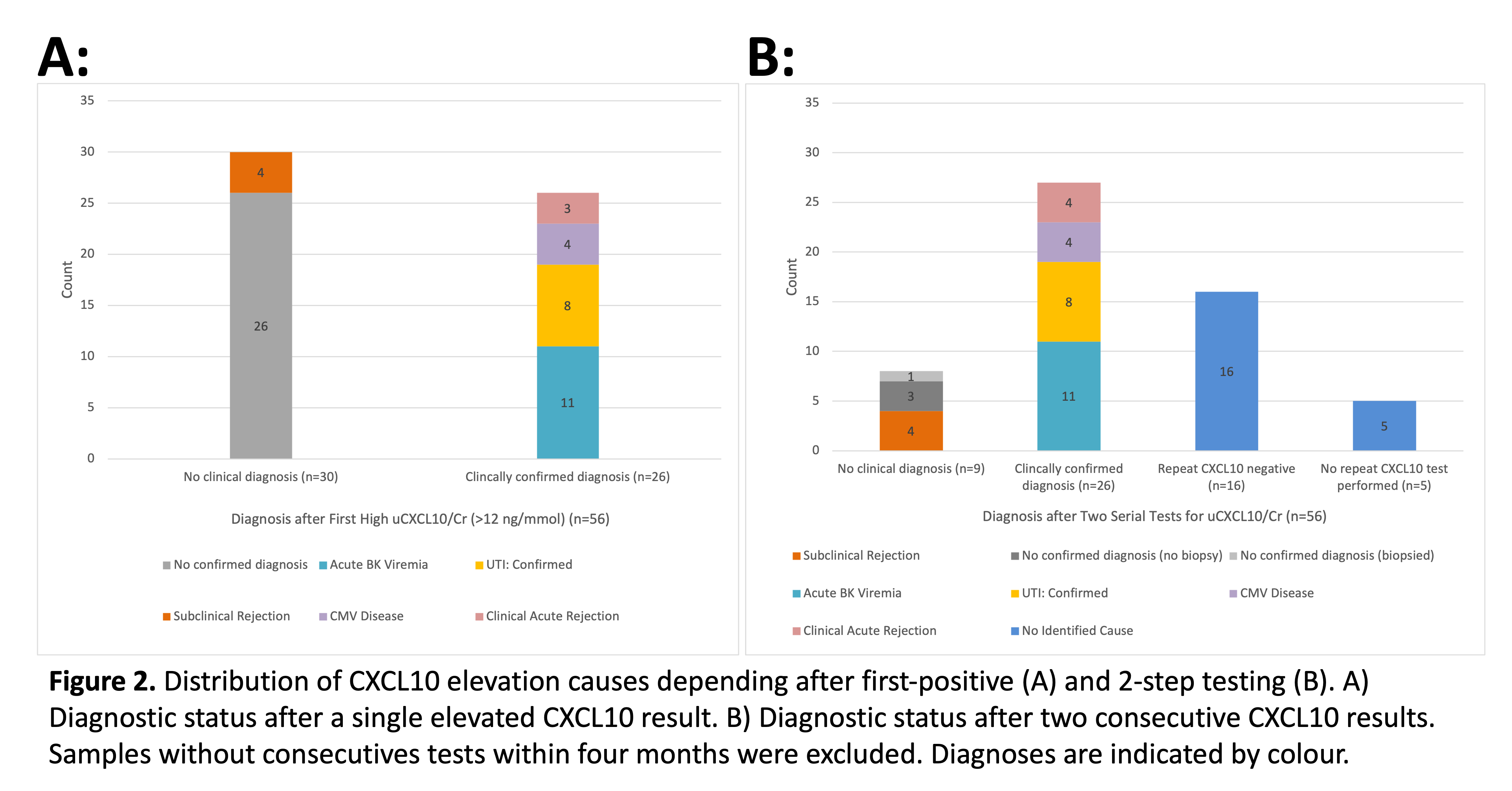
Significance: This work confirmed uCXCL10/Cr diagnostic thresholds to indicate biopsy for rejection risk (>90% specificity). A 2-step approach to diagnosis is superior to first-positive test for identifying alternate causes of graft inflammation, which improves yield for kidney biopsy to detect subclinical rejection.
Candice Wiedman. Monica Ho. BCCHR Summer Studentship Program.