
Ultrasound of muscle is a feasible tool to assess sarcopenia in children
Christopher Chu1, Patricia Acharya2, David Rigual2, Jennifer Dodge3,4, Norah Terrault3.
1Pediatric Gastroenterology, Hepatology and Nutrition, Children's Hospital Los Angeles, Los Angeles, CA, United States; 2Radiology and Imaging, Children's Hospital Los Angeles, Los Angeles, CA, United States; 3Gastrointestinal and Liver Disease, Keck Medical School of University of Southern California, Los Angeles, CA, United States; 4Population and Public Health Sciences, Keck Medical School of University of Southern California, Los Angeles, CA, United States
Introduction: The growth failure gap is a recognized shortcoming of the pediatric end-stage liver disease (PELD) score and argues that the PELD is a poor predictor of waitlist mortality1. Sarcopenia was identified as a potential novel and independent predictor of waitlist mortality and outcomes in adults with end-stage liver disease2-7. Ultrasound (US) has been used to diagnose sarcopenia in geriatric patients8-9, but no standard exists for the diagnosis of sarcopenia in pediatric patients. The aims of this pilot study are (1) to develop a method for characterizing muscle quality and quantity using US technology in pediatric patients and (2) to examine the correlation of these measures to body mass index (BMI) z-score with the goal of developing a tool for identifying sarcopenia amongst patients on the liver transplant waitlist.
Methods: Patients aged 2 months to 18 years with chronic liver disease (CLD) and healthy controls were recruited from March to August 2022 at Children’s Hospital Los Angeles. CLD was defined as history of persistent transaminitis for ≥ 60 days as a result of suspected or known primary hepatic dysfunction. Patients with history of liver transplantation or conditions that may affect the measurement of leg muscles such as neurologic or mechanical abnormalities were excluded. Height, weight and BMI were recorded for all participants. Three US measures for each recti femoris muscle were evaluated in triplicate for each participant; cross-sectional area (CSA), muscle thickness (MT) and echogenic intensity (EI). ImageJ (NIH, Washington DC) was used to calculate the average pixel intensity within the measured CSA for determining EI. US measures were assessed for reliability using intraclass correlation coefficients (ICCs) estimated from two-way mixed effects model for absolute agreement and means values compared by CLD vs healthy groups using linear regression. Pearson correlation coefficient was used to evaluate the relationship between BMI z-score and each muscle measure.
Results: A total of 119 patients were recruited (n=51, 42.9% CLD; 54.6% male; median age 5 years (IQR 1.7-10.2).
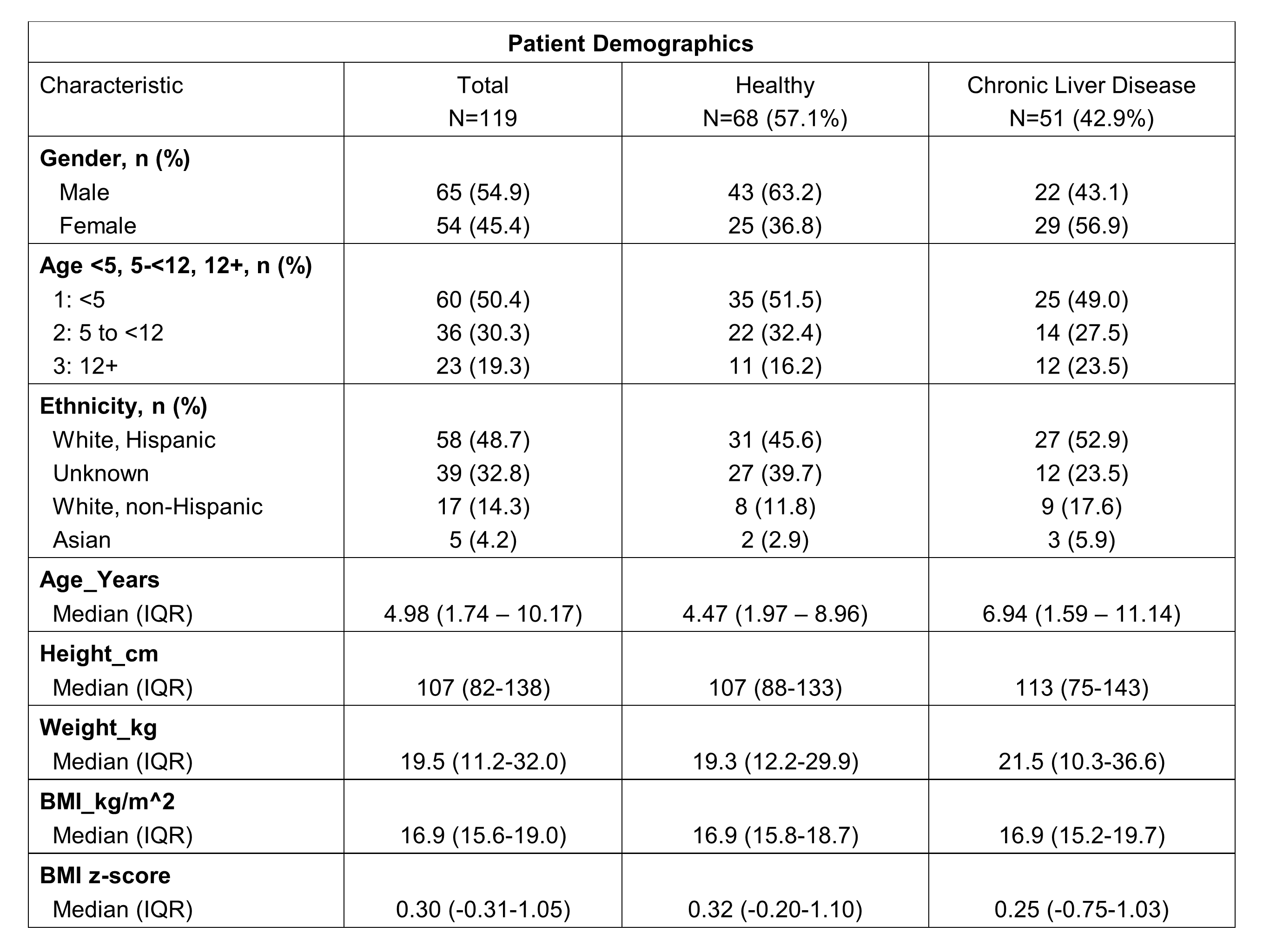
Excellent reliability (ICC>0.90) was demonstrated for CSA (0.994, CI 0.991-0.995), MT (0.971, CI 0.961-0.979) and EI (0.919, CI 0.887-0.942). BMI z-score was correlated with CSA and MT (r=0.30 and 0.34, respectively, all p = <0.001) but not EI (r=0.06, p=0.52). In multivariable linear regression, muscle measurements differed for CLD vs healthy participants for MT and EI adjusting for sex and age group but were not detectable for CSA.
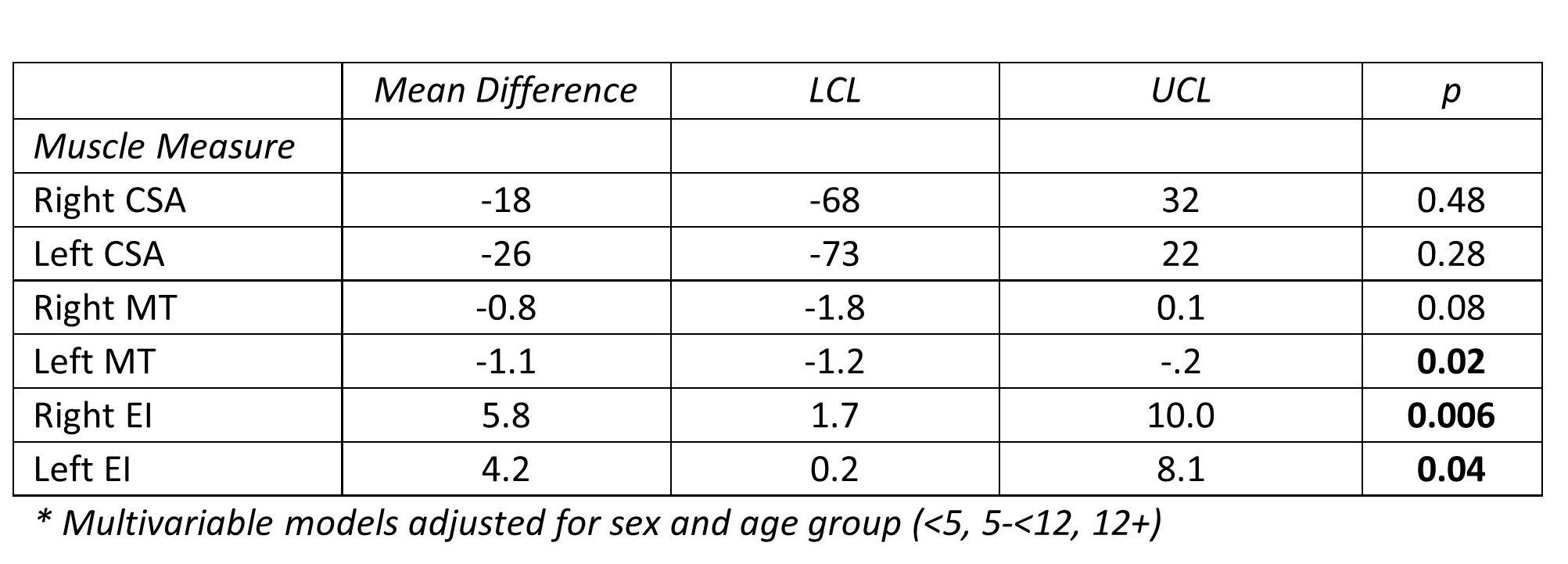
Conclusion: Ultrasound assessment of rectus femoris muscle is feasible and correlates significantly to BMI z-score. Muscle thickness and echogenic intensity significantly differed in CLD vs healthy participants in pediatric patients suggesting this may be a novel tool for assessing sarcopenia. Correlating muscle measures with waitlist outcomes among persons with CLD is an important future area of research.
OneLegacy Fellowship Training Grant. American College of Gastroenterology Clinical Research Pilot Award.
[1] Swenson SM, Roberts JP, Rhee S, Perito ER. Impact of the Pediatric End-Stage Liver Disease (PELD) growth failure thresholds on mortality among pediatric liver transplant candidates. Am J Transplant. 2019 Dec;19(12):3308-3318.
[2] Ooi PH, Hager A, Mazurak VC, Dajani K, Bhargava R, Gilmour SM, Mager DR. Sarcopenia in Chronic Liver Disease: Impact on Outcomes. Liver Transpl. 2019 Sep;25(9):1422-1438.
[3] Meeks AC, Madill J. Sarcopenia in liver transplantation: A review. Clin Nutr ESPEN. 2017 Dec;22:76-80.
[4] Uchiyama H. Sarcopenia in liver transplant recipients: its relevance to peritransplant morbidity and mortality. Hepatobiliary Surg Nutr. 2017;6(3):196-199.
[5] Krell RW, Kaul DR, Martin AR, et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19:1396–1402.
[6] Kallwitz ER. Sarcopenia and liver transplant: the relevance of too little muscle mass. World J Gastroenterol. 2015; 21:10982–10993.
[7] Golse N, Bucur PO, Ciacio O, et al. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transpl. 2017; 23:143–154.
[8] Perkisas, S., Baudry, S., Bauer, J. et al. Application of ultrasound for muscle assessment in sarcopenia: towards standardized measurements. Eur Geriatr Med. 9, 739–757 (2018).
[9] Stringer HJ, Wilson D. The Role of Ultrasound as a Diagnostic Tool for Sarcopenia. J Frailty Aging. 2018;7(4):258-261.