
High-dimensional profiling of pediatric immune responses to solid organ transplantation
Mahil Rao1,2, Meelad Amouzgar4, James T. Harden2,4, Mary G. Lapasaran2, Amber Trickey3, Brian Armstrong7, Jonah Odim8, Tracia Debnam8, Carlos O. Esquivel2,3, Sean C. Bendall5,6, Olivia M. Martinez2,3,5, Sheri M. Krams2,3,5.
1Pediatrics, Critical Care, Stanford University, Palo Alto, CA, United States; 2Transplant Immunology Lab, Stanford University, Palo Alto, CA, United States; 3Surgery, Abdominal Transplantation, Stanford University, Palo Alto, CA, United States; 4Immunology Graduate Program, Stanford University, Palo Alto, CA, United States; 5Program in Immunology, Stanford University, Palo Alto, CA, United States; 6Pathology, Stanford University, Palo Alto, CA, United States; 7Rho, Inc, Durham, NC, United States; 8National Institutes of Health, Bethesda, MD, United States
Purpose: Solid organ transplant is lifesaving for children with end-stage heart, liver, or kidney disease. Protection of the transplanted organ from rejection requires long-standing immunosuppression. However, despite our arsenal of immunosuppressive agents, 25% of pediatric transplant recipients have a rejection episode in the first-year post-transplant. The goal of our study was to identify novel and robust surrogate endpoints of allograft status.
Methods: Blood was obtained from pediatric recipients of liver, heart, kidney, or intestinal grafts enrolled at seven sites in the NIAID-sponsored Clinical Trials of Organ Transplantation in Children (CTOTC)-06, a prospective multi-institutional study. PBMCs from 52 children were analyzed, both from patients who had stable graft function (n=24) in the 12-month period before and after sample collection and patients who had an episode of biopsy-proven rejection (n=28) within 30 days after sample collection. These cohorts were well-balanced with respect to clinical characteristics. PBMCs were thawed, barcoded, pooled, and stained with 37 metal-conjugated antibodies against cell surface and intracellular proteins expressed in immune cell lineages and analyzed by single-cell mass cytometry (cytometry by time-of-flight; CyTOF). To identify differences in population frequency between stable and rejection cohorts, we computed cell-type proportions for each population by calculating the number of cells of each population divided by the total number of cells in the respective lineage.
Results: Principal component analysis revealed that allograft type is an important driver of differences in post-transplant immune composition. Liver and kidney recipients were more like each other and distinct from heart recipients. Heart recipients had higher proportions of CD4 EM, CD4 effector T cells, plasmablasts, and naïve B cells compared to liver or kidney recipients. We observed the same phenomenon using linear discriminant analysis, an independent method of analyzing differences between cohorts.
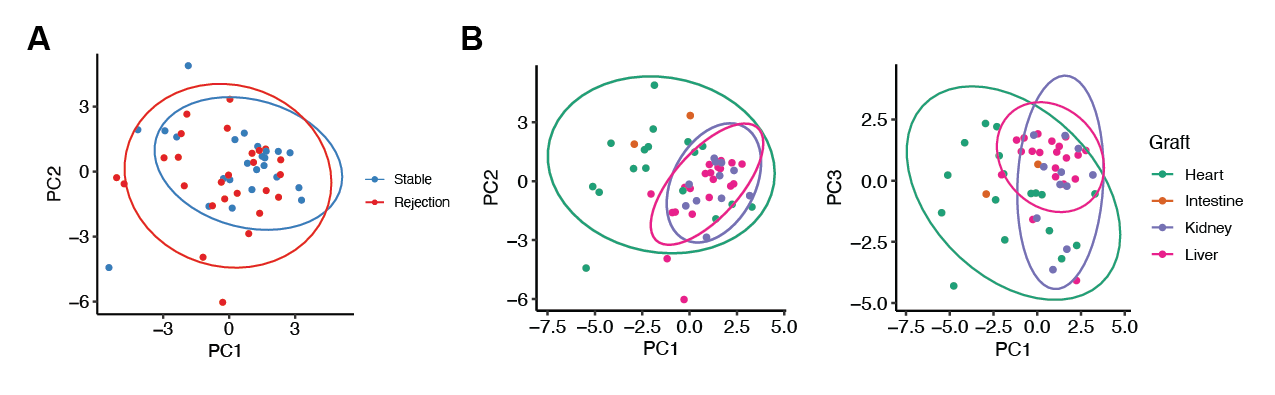
When controlling for differences in allograft type, we observed an increase in the proportion of CD8 naive and CD8 central memory cells in patients with graft rejection. In this same cohort, we also observed a decrease in the proportion of a novel T cell population (CD45+CD3+CD19-CD4+CD8-CD25loCD5+CD38-FoxP3loCD45RA-).

Conclusions: Single-cell CyTOF analysis identified distinct differences in immune response based on allograft status and identified a population whose abundance is associated with increased graft stability. Additional studies will determine if this T cell population can be harnessed to predict or prevent allograft rejection.
National Institutes of Health UO1 AI104342 [C.O.E.]. National Institutes of Health U01 AI1359590 [S.M.K.]. Stanford Maternal and Child Health Research Institute Clinical Trainee Award [M.R.]. Stanford Transplant and Tissue Engineering Center of Excellence [M.R.]. Stanford University Jackson Vaughan Critical Care Research Fund [M.R.]. Stanford University Immunology Training Grant T32 AI007290_37 [M.A.].