Determining the role of complement pro-inflammatory factors and regulatory proteins in antibody-mediated rejection (ABMR) in paediatric kidney transplant recipients (pKTR)?
Rishil Patel1,2, Olumide Ogunbiyi1, Katherine Swarbrick1, Thivya Sekar1, Nithiakishna Selvathesan3, Natalie Wyatt1, Stephen D. Marks1,2.
1Pediatric Nephrology, Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom; 2Pediatric Nephrology, University College London Great Ormond Street Institute of Child Health, London, United Kingdom; 3Paediatric Nephrology, The Hospital for Sick Children, Toronto, ON, Canada
Introduction: The accurate and prompt diagnosis of ABMR in pKTR is vital in allowing the early initiation of treatment to prevent renal allograft dysfunction and loss. The important role of complement activation in renal transplant immunology and rejection has been described, especially when some pKTR can have circulating peripheral donor specific antibodies for years. Membrane-bound complement regulatory proteins (mCRPs), such as CD46, CD55, and CD59, are expressed throughout the body to prevent over-activation of the complement system. Pro-inflammatory factors such as spleen tyrosine kinase (Syk) and C3d promote leucocyte recruitment and activation, and enhance the immune response. There are limited data in the paediatric population identifying the presence and deposition of specific complement pro-inflammatory and inhibitory factors in suspected ABMR.
Methods: Retrospective, single-centre study of percutaneous renal transplant biopsies from 55 pKTR with donor-specific antibodies (DSA) and a histological diagnosis of ABMR. Following the optimization of the above antibodies, the tissue materials of these 55 patients were retrieved from GOSH archives for IHC staining with CD46, CD55, CD59, SYK and C3D. The renal tissues were formalin-fixed-paraffin-embedded (FFPE) and sectioned at 3 microns thickness.
The slides were then scored using a semi-quantitative scoring system on strength of staining: 0 = no significant staining, 1+ = patchy staining and 2+ = strong staining; and location = glomerular capillaries, proximal or distal tubules.
Results: There was no evidence of CD55 staining in any biopsies.
CD59 staining was positive in 96% of biopsies, with stronger expression in peritubular capillaries, and patchy staining in the proximal and distal tubules.
CD46 showed positive staining in 91% of biopsies, with patchy staining (49%) in the basement membrane and in the proximal and distal tubules.
C3D staining showed strongly positive staining in 87% of biopsies, mainly in glomerular capillaries, and some patchy staining in the tubules.
SYK staining was positive in all biopsies, predominantly strongly positive (80%) and confined to the distal tubules.
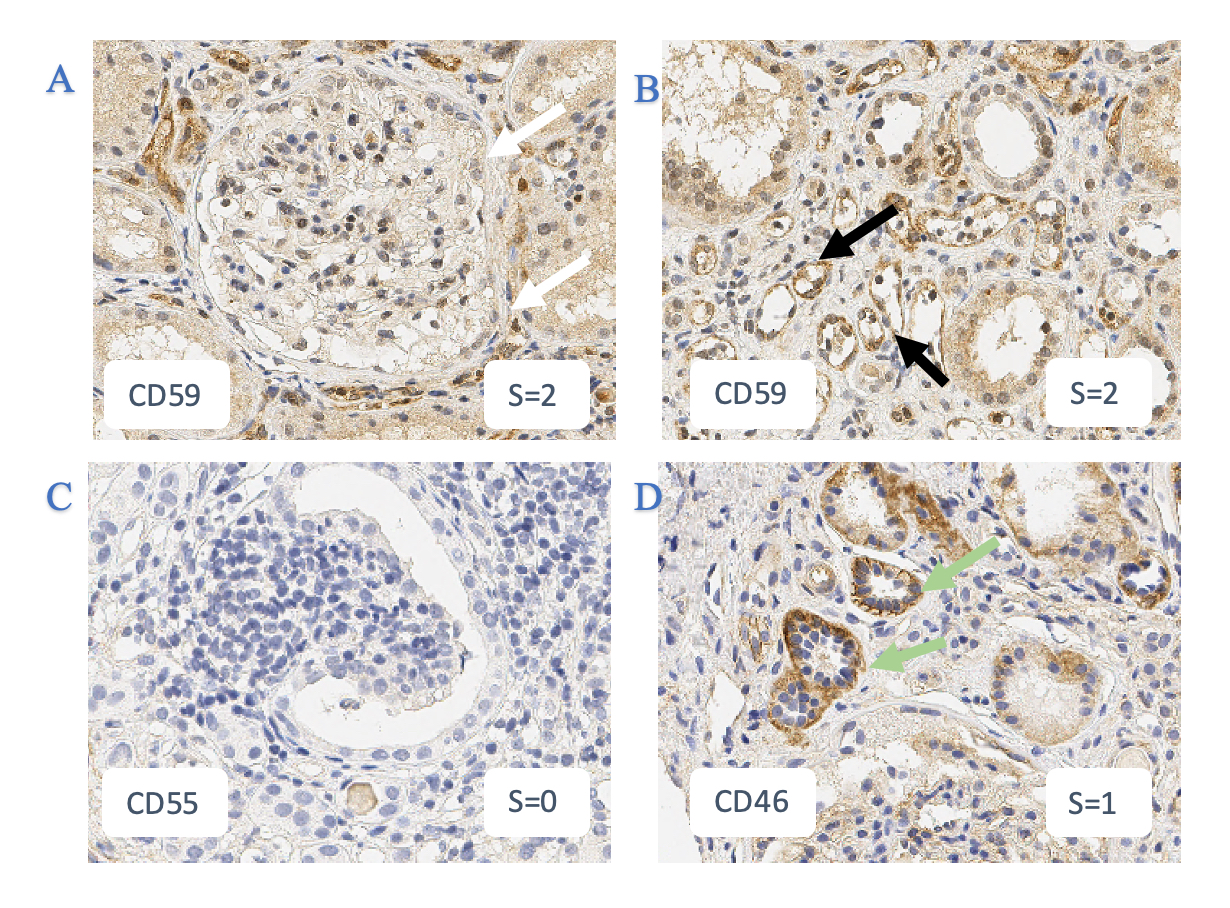
FIGURE 1
White arrows indicate glomeruli, black arrows indicate peritubular capillaries, green arrows indicate proximal and distal tubules
Semi-quantitative scores (0-2) indicated in the lower right corner
Conclusions Our results suggest the proximal complement regulator CD55 is underactive in ABMR. Positive CD59, C3D, SYK and CD46 staining was observed in biopsies of pKTR with ABMR, with varying strength and localisation confirming the important role of complement in ABMR. Further studies are warranted to evaluate their use as diagnostic and prognostic tools in ABMR, and to determine the potential role for complement targeted therapies.
[1] Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14(2):255-71.
[2] Horwitz JK, Chun NH, Heeger PS. Complement and Transplantation: From New Mechanisms to Potential Biomarkers and Novel Treatment Strategies. Clin Lab Med. 2019;39(1):31-43.
[3] de Boer ECW, van Mourik AG, Jongerius I. Therapeutic Lessons to be Learned From the Role of Complement Regulators as Double-Edged Sword in Health and Disease. Front Immunol. 2020;11:578069.
[4] Kieslich CA, Morikis D. The two sides of complement C3d: evolution of electrostatics in a link between innate and adaptive immunity. PLoS Comput Biol. 2012;8(12):e1002840
[5] Goldberg BS, Ackerman ME. Antibody-mediated complement activation in pathology and protection. Immunol Cell Biol. 2020;98(4):305-17.
[6] Geller A, Yan J. The Role of Membrane Bound Complement Regulatory Proteins in Tumor Development and Cancer Immunotherapy. Front Immunol. 2019;10:1074.
[7] Schinstock CA, Bentall AJ, Smith BH, Cornell LD, Everly M, Gandhi MJ, et al. Long-term outcomes of eculizumab-treated positive crossmatch recipients: Allograft survival, histologic findings, and natural history of the donor-specific antibodies. Am J Transplant. 2019;19(6):1671-83.