Paediatric Gastroenterology/Hepatology
University of Witwatersrand, Faculty of Health sciences
Charlotte Maxeke Johannesburg Academic Hospital
Epstein Barr Virus risk stratification as a tool to predict post-transplant lymphoproliferative disorder in pediatric liver transplant recipients
Priya Walabh1,2,4,5, Sechaba T. Palweni3,4,6, Anja Meyer4,6, Pravina Walabh8, Christina Hajinicolaou1,2,7.
1Paediatrics and Child Health, University of Witwatersrand, Faculty of Health Sciences, Johannesburg, South Africa; 2Paediatric Gastroenterology, Hepatology and Nutrition, University of Witwatersrand, Faculty of Health Sciences, Johannesburg, South Africa; 3Surgery, University of Witwatersrand, Faculty of Health Sciences, Johannesburg, South Africa; 4Gauteng Solid Organ Transplant Governance, Gauteng Department of Health, Johannesburg, South Africa; 5Paediatric Gastroenterology, Hepatology and Nutrition, Charlotte maxeke Johannesburg Academic Hospital, Johannesburg, South Africa; 6Surgery, Charlotte maxeke Johannesburg Academic Hospital, Johannesburg, South Africa; 7Divisional Head Paediatric Gastroenterology, hepatology and nutrition, Chris Hani Baragwanath Hospital, Johannesburg, South Africa; 8Science, University of Cape Town, Johannesburg, South Africa
Introduction: Post-transplant lymphoproliferative disorder (PTLD) is a clinically heterogenous potentially fatal complication of pediatric liver transplant (PLT) recipients with an incidence of 10 to 20% in liver transplant recipients and a mortality which has improved over recent years with the advent of improved therapeutic options. Our aim was to determine the incidence, complications and associated factors for developing post-transplant lymphoproliferative disorders in pediatric liver transplant recipients at Wits Donald Gordon Medical Centre from January 2012 to August 2019. Permission for this study was obtained from HREC at University of Witwatersrand.
Methods: Retrospective record review of all 150 pediatric liver transplant recipients was done. Data collected included clinical and demographic data including Epstein Barr Virus (EBV), Cytomegalovirus (CMV) serology of donor and recipients, EBV viral loads, presentation, histology, treatment and outcome of recipients with PTLD.
Results: The median age at transplant was 29.2 months (IQR 15.6-58.4) and follow up was 30 months (IQR 13- 55). The incidence of PTLD in our cohort was 17/150 (11.3%). The incidence of PTLD in our cohort was 17/150 (11.3%). The median weight of recipients who developed PTLD was 10.0 kg (IQR 7.5-12.2) which was significantly lower (P = 0.006) at the time of transplant compared to those patients who didn’t develop PTLD. Patients who developed PTLD were also significantly younger (<18 months) (P = 0.002) and 15/17 (88.2%) were in the high-risk category (P= 0.003).
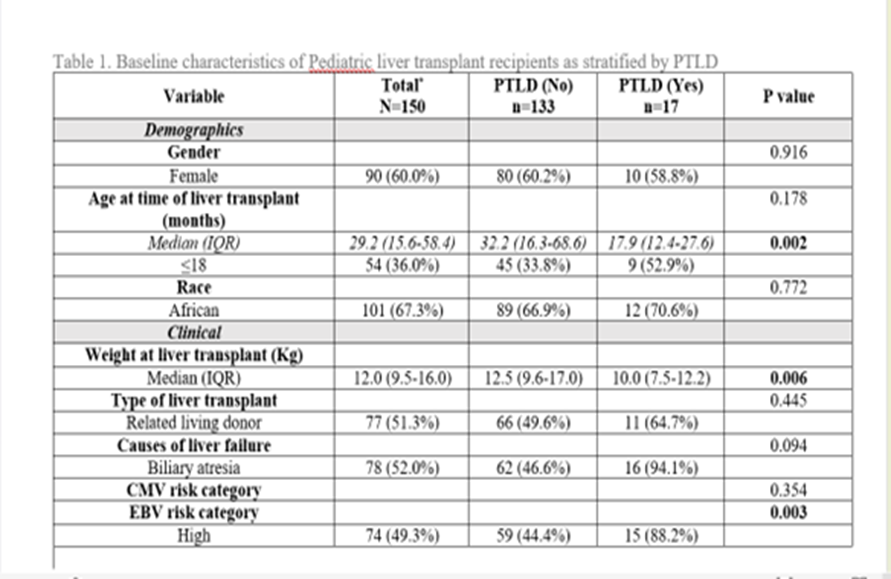
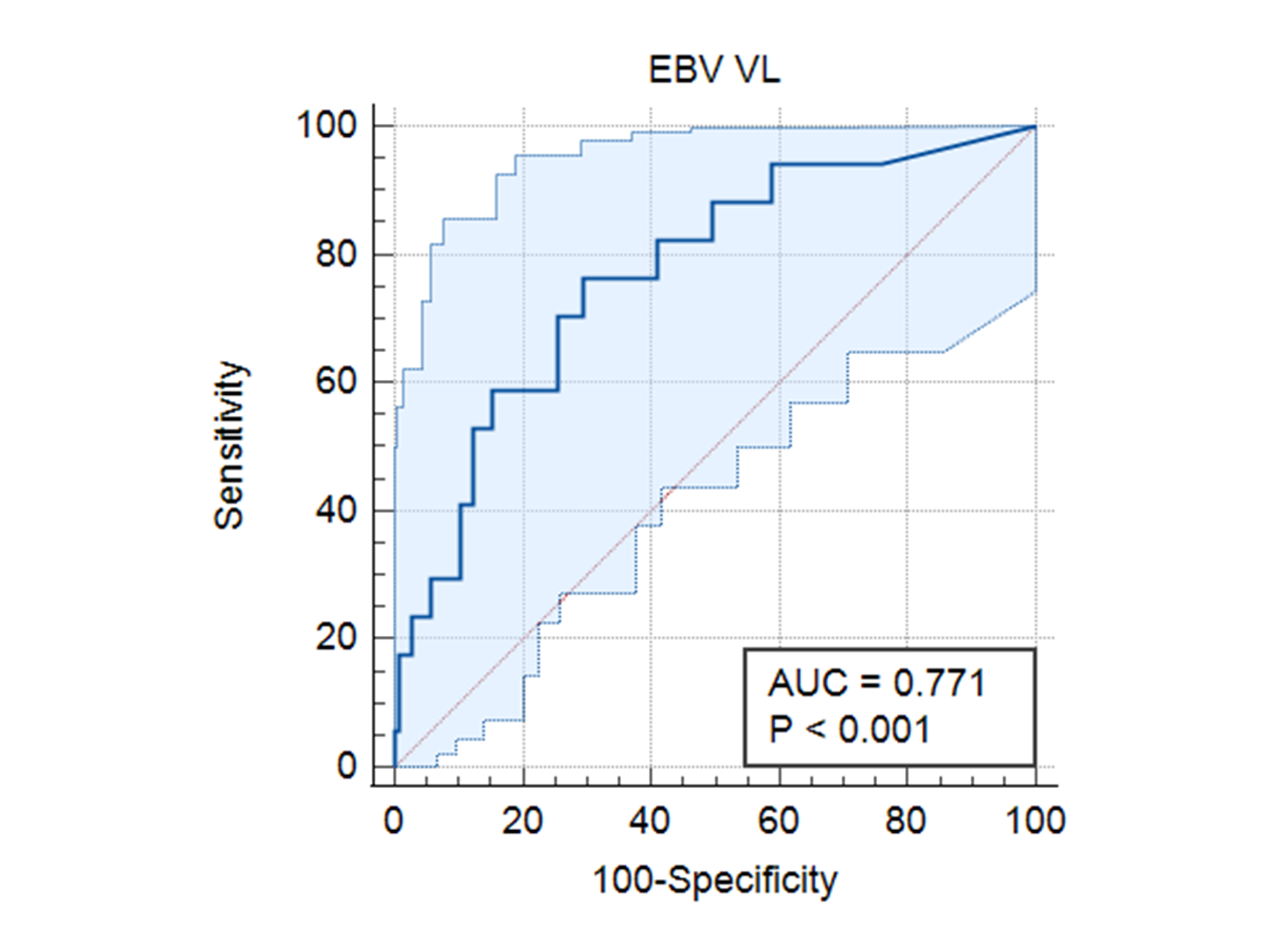
Although there was no statistically significant association between CMV prophylaxis (intravenous ganciclovir and oral valganciclovir) and PTLD, we found that none of the patients who received CMV prophylaxis developed PTLD. A cut-off EBV viral load >57136 cop/ml (Log 5.1) using the ROC curve showed a specificity of 70.1 % and a sensitivity of 76.5% for developing PTLD [ AUC 0.77 95% CI (0.69- 0.84) P < 0.0001].
Conclusion: EBV stratification and viral loads can be helpful in predicting PTLD. Malnutrition parameters need to be explored in future studies as weight significantly predicted development of PTLD in PLT recipients in our cohort.
References:
[1] Dharnidharka VR, Webster AC, Martinez OM, Preiksaitis JK, Leblond V, Choquet S. Post-transplant lymphoproliferative disorders. Nat Rev Dis Prim. 2016;2. doi:10.1038/nrdp.2015.88
[2] Ramos-Gonzalez G, Crum R, Allain A, et al. Presentation and outcomes of post-transplant lymphoproliferative disorder at a single institution pediatric transplant center. Pediatr Transplant. 2022;(March):1-7. doi:10.1111/petr.14268
[3] Kamdar KY, Rooney CM, Heslop HE. Posttransplant lymphoproliferative disease following liver transplantation. Curr Opin Organ Transplant. 2011;16(3):274-280. doi:10.1097/MOT.0b013e3283465715
[4] Éboli LP de CB, Tannuri ACA, Tannuri U. Seropositivity for cytomegalovirus and PCR-EBV monitoring: Protective factors for posttransplant lymphoproliferative disorder in pediatric liver transplant. Pediatr Transplant. 2022;26(4):1-9. doi:10.1111/petr.14226
Lectures by Priya Walabh
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sun-26 10:00 - 11:00 |
Ethical/Psychosocial and Economical Issues | Disparity in organ distribution in South Africa | Hill Country CD |
|
Sat-25 18:00 - 19:15 |
P5- Infectious Diseases Posters | Epstein Barr Virus risk stratification as a tool to predict post-transplant lymphoproliferative disorder in pediatric liver transplant recipients | Zilker 1-2 |
