The Children's Hospital of Philadelphia
Single center experience of surveillance biopsies in pediatric kidney transplants
Nathaniel Zona1, Jon Savant2, Lauren Galea2, Jennifer Hewlett2, Sonya Lopez2, Benjamin Laskin2, Christopher LaRosa2, Selasie Goka2, Sandra Amaral2, Bernarda Viteri2.
1Kalamazoo College, Kalamazoo, MI, United States; 2Department of Pediatrics, Children's Hospital of Philadelphia, Philadelphia, PA, United States
Introduction: Acute rejection remains a significant risk factor for allograft loss in pediatric renal transplant recipients. Some evidence suggests that identifying subclinical rejection through surveillance biopsies improves allograft function and survival. There are no strong patient demographic patterns associated with increased risk of subclinical rejection. Furthermore, appropriate pediatric surveillance biopsy time intervals have yet to be standardized to minimize risk and maximize clinical outcomes. In January 2020, we began a surveillance biopsy protocol at our single, large academic center.
Methods: We conducted a retrospective chart review of all renal allograft surveillance biopsies performed since Jan 2020. Same-day surveillance biopsies were performed at six, twelve-, and twenty-four-months post-transplant. Patients with anatomical concerns, bleeding dyscrasias, multi-organ transplant, or general anesthesia requirements were excluded. For cause biopsies were excluded. Clinical and demographic characteristics were collected from the medical chart. Biopsies were assessed by a renal pathologist and classified by Banff 2017 criteria. Clinical and demographic characteristics and rates of subclinical rejection and acute rejection were examined using descriptive statistics.
Results: 29 subjects had a total of 47 surveillance biopsies to date. The mean age at transplant was 12.2 years (± 5.0 years) and 18 (62%) were male. Subclinical inflammation or rejection was identified in 10 (21.3%) surveillance biopsies of 9 subjects (1 subject had 2 subsequent positive biopsies, first positive accounted in analysis). Subclinical inflammation or rejection was found in 3 (13.6%) out of 22 six-month surveillance biopsies (IQR: 6.1–7.0 months) and in 7 (38.9%) out of the 18 twelve-month surveillance biopsies (IQR: 11.9–13.9 months). The 7 surveillance biopsies performed at the twenty-four-month interval were negative for both; data collection is still ongoing for this group. Therapy plan changes were made in response to 9 of 10 (90%) positive biopsy findings and 6 of 37 (16.2%) relevant negative findings on biopsy. We experienced 6 (12.8%) minor complications in all surveillance biopsies (significant hematoma n=2; gross hematuria n=4). No major complications were experienced.
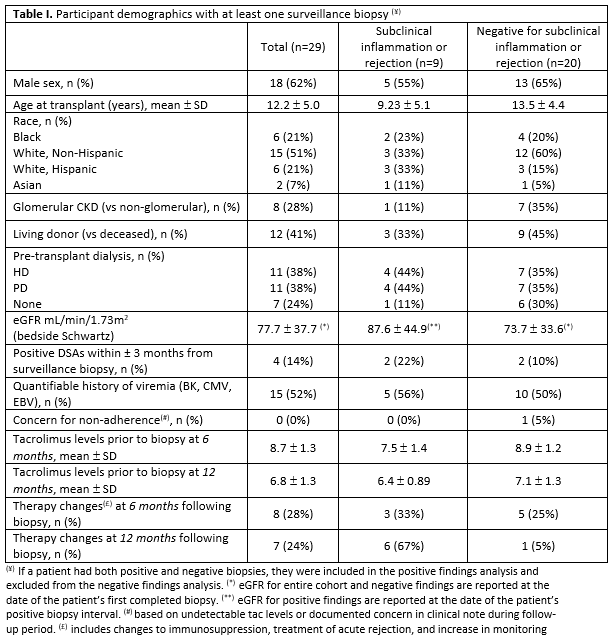
Conclusions: Surveillance biopsies were performed with minimal complication as a same-day procedure and informed treatment plans, with changes to immunosuppression in 14 subjects. We found no differences in DSA, history of nonadherence, or traditional risk factors for rejection between patients with or without subclinical rejection. These results highlight the added value of surveillance biopsies. Of note, biopsies at 24 months to date have been unremarkable. An extended follow up period is necessary to evaluate the long-term outcomes associated with intervention. Further studies with larger samples sizes are needed to inform the best timing.
Lectures by Nathaniel Zona
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-25 18:00 - 19:15 |
P6- Kidney Posters | Single center experience of surveillance biopsies in pediatric kidney transplants | Zilker 1-2 |
