Observational Case Series on Utilizing C1 Esterase Inhibitor for Prevention of Delayed Graft Function In Pediatric Renal Transplantation
Helen Pizzo1, Jonathan Garrison2, Kathyln Lim2, Stanley C. Jordan2, Dechu Puliyanda1.
1Pediatric Nephrology, Cedars-Sinai Medical Center, Los Angeles, CA, United States; 2Transplant Immunology Laboratory and Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Background: Delayed graft function (DGF) mediated by ischemia-reperfusion injury (IRI) is associated with an increased risk for rejection, and decreased long-term allograft function and survival.1-3 Graft survival at 1 year in patients with DGF ranged between 70.5%-91% depending on duration of DGF.1 Therefore, prevention of DGF in those at risk is paramount. Experimental studies suggest that the classical and mannose binding lectin pathways play an important role in IRI; these pathways can be inhibited by C1 esterase inhibitor (C1INH). Here, we report our center’s experience with the use of C1INH in pediatric renal transplant recipients at risk for delayed graft function. We highlight the duration of index hospitalization, the need for dialysis in the first week after transplant, rejection, and serial eGFR up to 2-years after transplant.
Methods: Between January 2020 and August 2022, a total of 4 out of 18 pediatric renal transplant recipients received C1INH (20 units/kg) due to an increased risk for DGF. 3 received kidneys that were donated after cardiac death (DCD). 1 received C1INH on post-operative day 1 for prolonged warm ischemia time and lack of perfusion to the allograft from renal artery thrombosis discovered on renal ultrasound at 1-hour post-transplant.
Results: Table 1 highlights the recipient and donor characteristics. Crossmatches were negative and there were no pre-formed donor-specific antibodies. Table 2 illustrates post-transplant outcomes including the duration of index hospitalization, the need for dialysis in the first week, and rejection. By 2 weeks after transplant, 2 of 4 had eGFR > 60 ml/min/1.73m2 and by 3 months 3 of 4 had eGFR > 90 ml/min/1.73m2 (Table 3). No patients experienced graft failure, or meningococcal infection.
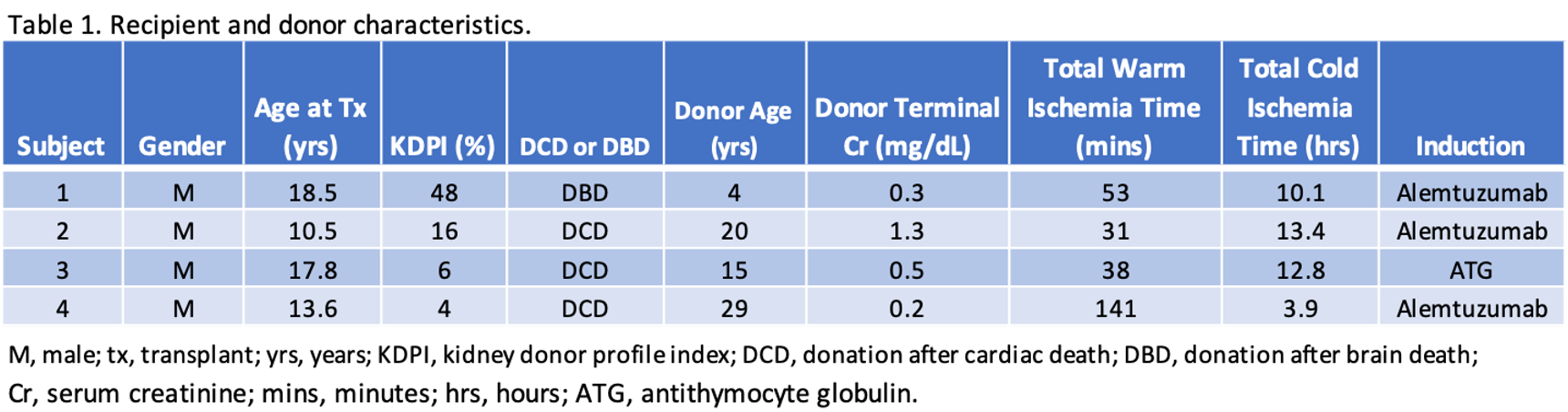
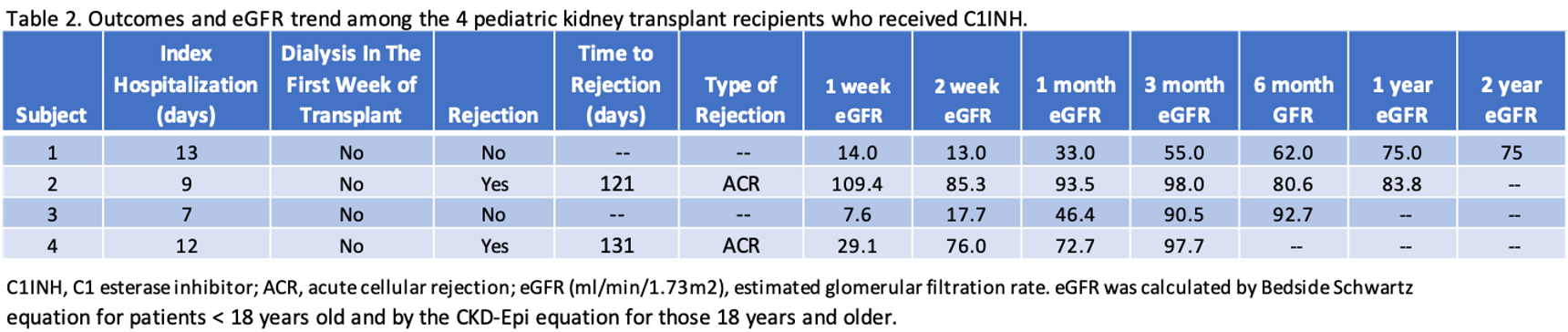
Conclusion: In our limited observational case series, C1INH appears to be a potential therapeutic for decreasing the incidence of DGF in at-risk pediatric renal transplant recipients. Utilization of C1INH may expand the use of kidneys from DCD donors and therefore broaden the donor pool for pediatric recipients. Future studies should include a larger sample size with longer follow-up and a control group to better delineate outcomes associated with the use of C1INH in pediatric transplant recipients at risk for DGF.
References:
[1] 1. de Sandes-Freitas TV, et al. Prolonged Delayed Graft Function Is Associated with Inferior Patient and Kidney Allograft Survivals. PLoS One. 2015 Dec 17;10(12):e0144188. doi: 10.1371/journal.pone.0144188.
[2] 2. Maia LF, et al. Effect of Delayed Graft Function on the Outcome and Allograft Survival of Kidney Transplanted Patients from a Deceased Donor. Transplant Proc. 2021 Jun;53(5):1470-1476. doi: 10.1016/j.transproceed.2021.04.002.
[3] 3. Helfer MS, et al. Long-term effects of delayed graft function duration on function and survival of deceased donor kidney transplants. J Bras Nefrol. 2019 Apr-Jun;41(2):231-241. doi: 10.1590/2175-8239-jbn-2018-0065. Epub 2018 Oct 4.
Lectures by Dechu Puliyanda
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-25 18:00 - 19:15 |
P6- Kidney Posters | Observational Case Series on Utilizing C1 Esterase Inhibitor for Prevention of Delayed Graft Function In Pediatric Renal Transplantation | Zilker 1-2 |
|
Tue-28 10:40 - 11:40 |
Immunosuppression | Newer antibodies therapies in the pipeline | Hill Country CD |
|
Sun-26 12:30 - 13:30 |
Paradigm Shifts in Pediatric Surveillance Testing: AlloSure and AlloMap in Action | Clinical Utility of AlloSure in the Kidney Transplanted Pediatric Population | Zilker 3-4 |
