
Burkhard Tönshoff, MD, PhD, is professor of pediatrics and pediatric nephrology at the University Children’s Hospital Heidelberg, Germany; Vice Chairman of the Department of Pediatrics I (General Pediatrics, Neuropediatrics, Metabolism, Gastroenterology, and Nephrology); Medical Director of the Pediatric Kidney Transplantation Program. His current research focuses on various issues in acute and chronic kidney disease and renal transplantation, such as studies on the pharmacokinetics, efficacy and safety of novel immunosuppressive drugs in pediatric renal transplant recipients, optimization of immunosuppressive therapy by therapeutic drug monitoring and immune monitoring, prevention of infectious and other complications after renal transplantation, biomarker-guided minimization of immunosuppressive therapy and the impact of donor-specific HLA and non-HLA antibodies on graft histology and function. In the year 2009 he founded the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN; www.certain-registry.eu) as a multicenter research network and platform built on a novel, web-based registry. Other research activities focus on the pathophysiology and therapy of the nephrotic syndrome, biomarker research for acute kidney injury, the role of endothelial progenitor cells and mesenchymal stem cells in various pediatric kidney diseases and the role of the gut microbiome in solid organ transplantation.
Awards and nominations: Eurotransplant Kidney Advisory Committee. Scientific Steering Committee der DZIF Transplantationskohorte e.V.. Chair, Working Group “Renal Transplantation” of the European Society for Paediatric Nephrology (ESPN) and of the German Society for Paediatric Nephrology (GPN). Council of the International Pediatric Transplant Association (IPTA); 2015 - 2017 President of IPTA. Since 2018 Editor-in-Chief of the journal “Pediatric Transplantation”, the official journal of IPTA.
Humoral immune response and live-virus neutralization of the SARS-CoV-2 omicron (BA.1) variant after standard COVID-19 mRNA vaccination in immunosuppressed children, adolescents and young adults with chronic kidney disease
Maximilian Stich1, Veronica Di Cristanziano2, Burkhard Tönshoff1, Lutz Thorsten Weber3, Jörg Dötsch3, Marian Theodor Rammer3, Susanne Rieger1, Eva Heger2, Sven F. Garbade1, Kathrin Burgmaier3, Louise Benning4, Claudius Speer4, Sandra Habbig3, Sophie Haumann3.
1Departement of Pediatrics I, University Children's Hospital Heidelberg, Heidelberg, Germany; 2Institute of Virology, University Hospital Cologne and University of Cologne, Cologne, Germany; 3Department of Pediatrics, University Hospital Cologne and University of Cologne, Cologne, Germany; 4Department of Nephrology, University of Heidelberg, Heidelberg, Germany
Background: Data on the humoral immune response to standard COVID-19 vaccination are scarce in adolescents and especially in children below 12 years of age with chronic kidney diseases (CKD) including pediatric kidney transplant recipients (KTR).
Methods: We therefore investigated in a two-center retrospective observational cohort study the humoral immune response including live-virus neutralization against the SARS-CoV-2 omicron (BA.1) variant after standard COVID-19 mRNA vaccination in 123 patients with CKD aged 5–30 years at the University Children’s Hospitals in Heidelberg and Cologne (Germany) between April 2021 and April 2022.
Results: Humoral immune response differed significantly (P=0.008) among three patient cohorts: 29 of 77 (37.7%) KTR, 5 of 26 (19.2%) of patients with CKD on immunosuppressive therapy, and 1 of 20 (5%) CKD controls did not respond. The magnitude of the humoral immune response in KTR (median 117 [IQR, 0–769] BAU/mL) was 9-fold lower (P<0.001) than in CKD controls (median 1046 [IQR, 470–2735] BAU/mL). In the total cohort (n=123) and KTR cohort (n=77) children aged 5–11 years had a comparable rate and degree of immune response as adolescents and young adults despite lower vaccine doses (10µg vs. 30µg BNT162b2).
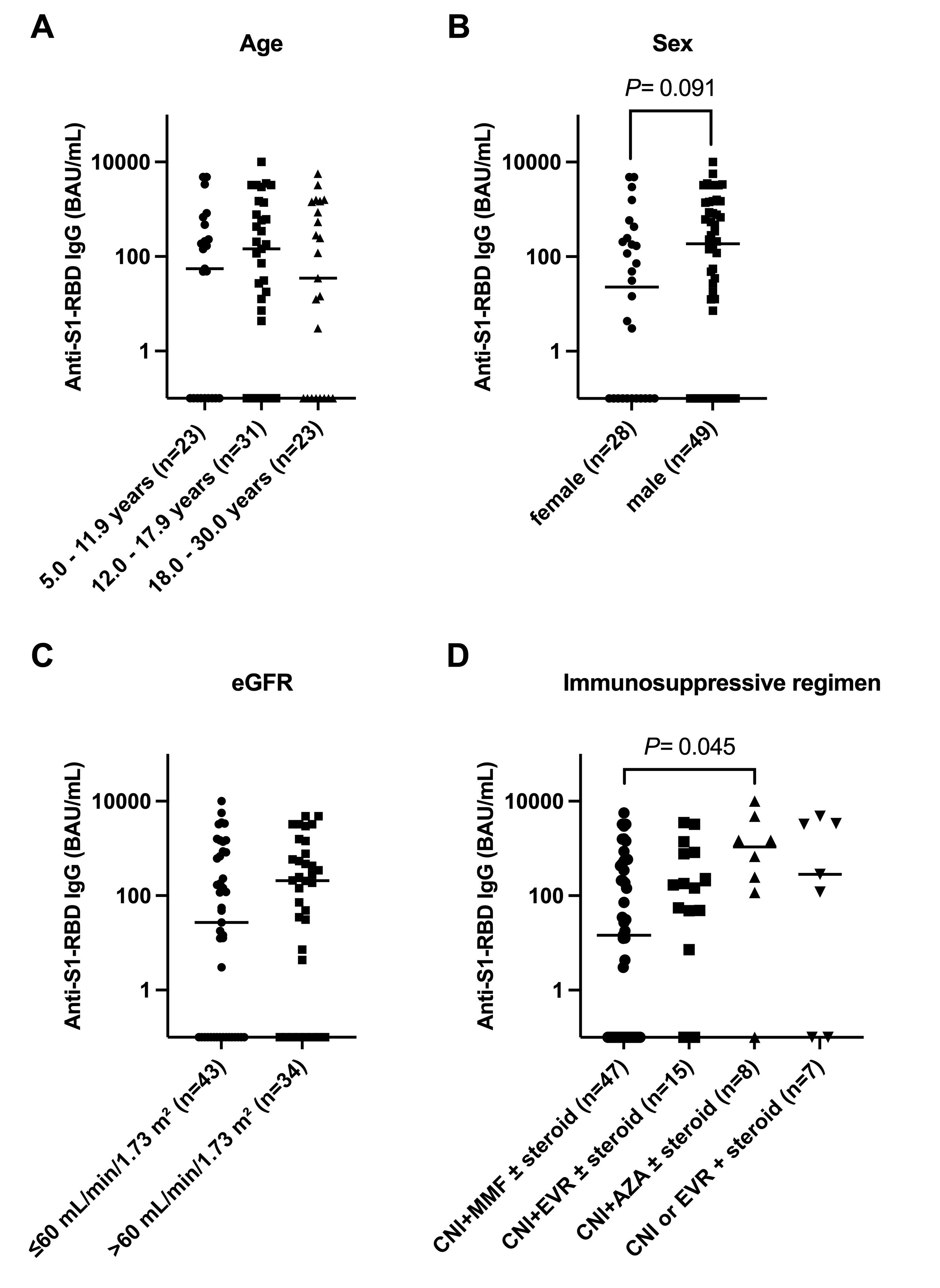
Independent risk factors for non-response in the full cohort (n=123) were treatment with two (OR, 9.24; 95% CI, 1.61–175.7) or three or more immunosuppressants (OR, 17.07; 95% CI, 2.91–328.0) and in the subgroup of KTR patients (n=77) an eGFR < 60 mL/min/1.73 m² (OR, 3.44; 95% CI, 1.16 – 11.4), female sex (OR, 3.11; 95% CI, 1.05 – 10.0) and an immunosuppressive regimen in conjunction with MMF compared with everolimus (OR, 0.15; 95% CI, 0.02 - 0.72) or azathioprine (OR, 0.09; 95% CI, 0.01-0.62). After the third vaccination, 8 of 15 (53.3%) previously seronegative KTR turned seropositive but the magnitude of the humoral immune response was lower than in those with a response after two vaccinations (anti-S1-RBD IgG, 120.1 [IQR, 50.8 – 340.6] BAU/mL vs. 506.2 [IQR, 144.7 – 1583] BAU/mL, p=0.036). Of patients with available serum samples and a BAU of ≥ 100/mL, only 10 of 34 (29.4%) showed any neutralizing activity (ID50 ≥1:10) against the omicron (BA.1) variant with a significant positive correlation between the anti-SARS-CoV-2-IgG titer and the corresponding neutralizing activity (R = 0.714, p < 0.001). A ROC analysis revealed an optimal cut-off at 1411 BAU/mL (ROC-AUC, 0.74; 95% CI, 0.54 – 0.95, P= 0.028) with 8 of 16 (50%) patients above but only 2 of 18 (11%) patients below this cut-off showing any live-virus neutralization activity against omicron (BA.1)
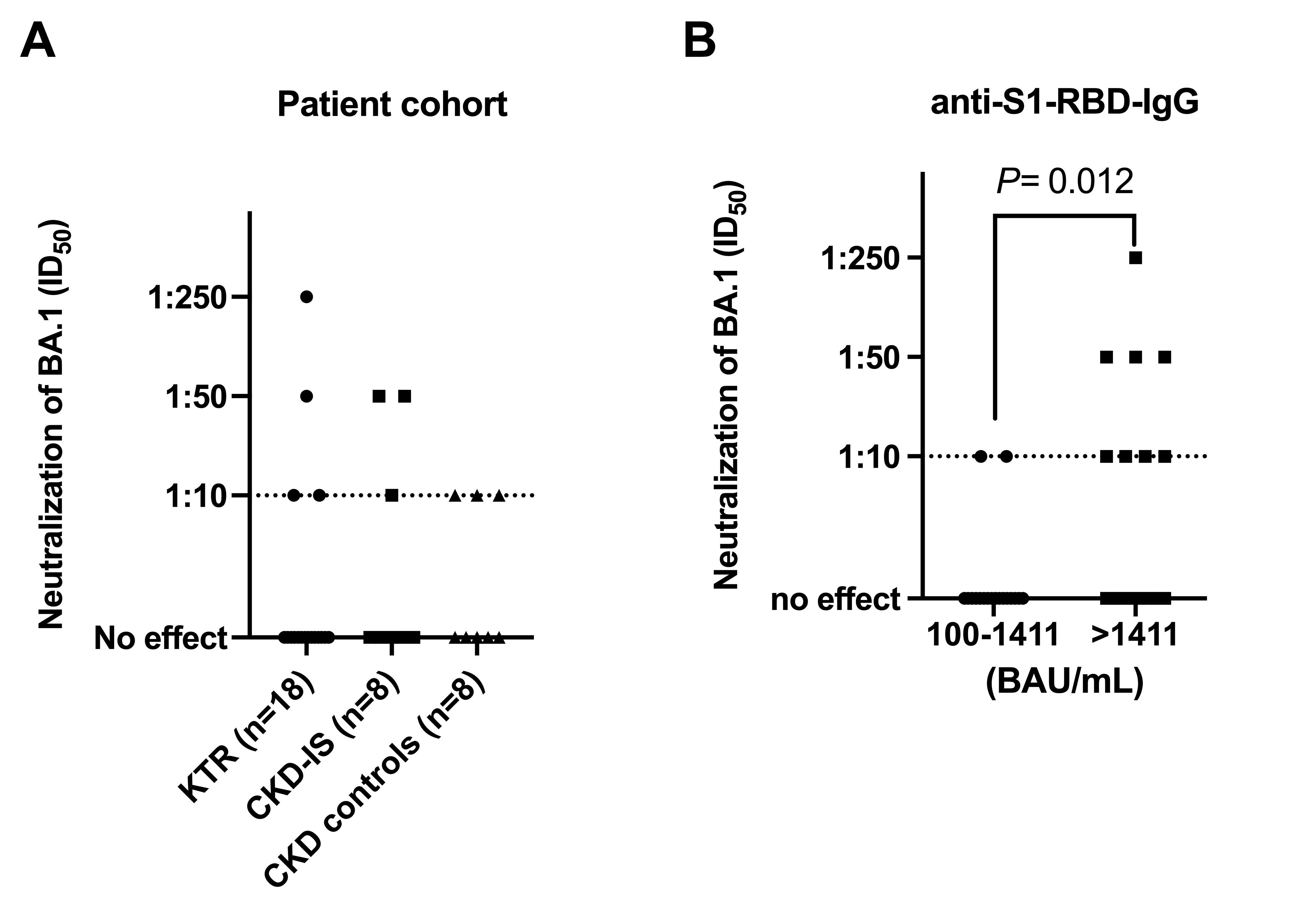 .
.
Conclusion: A standard mRNA vaccine regimen in immunosuppressed children and adolescents with CKD and KTR elicit an attenuated humoral immune response. Live-virus neutralization against the omicron variant is achieved in only approx. 10% of immunosuppressed pediatric CKD and KTR patients when extrapolating these data to the entire patient cohort.