
Burkhard Tönshoff, MD, PhD, is professor of pediatrics and pediatric nephrology at the University Children’s Hospital Heidelberg, Germany; Vice Chairman of the Department of Pediatrics I (General Pediatrics, Neuropediatrics, Metabolism, Gastroenterology, and Nephrology); Medical Director of the Pediatric Kidney Transplantation Program. His current research focuses on various issues in acute and chronic kidney disease and renal transplantation, such as studies on the pharmacokinetics, efficacy and safety of novel immunosuppressive drugs in pediatric renal transplant recipients, optimization of immunosuppressive therapy by therapeutic drug monitoring and immune monitoring, prevention of infectious and other complications after renal transplantation, biomarker-guided minimization of immunosuppressive therapy and the impact of donor-specific HLA and non-HLA antibodies on graft histology and function. In the year 2009 he founded the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN; www.certain-registry.eu) as a multicenter research network and platform built on a novel, web-based registry. Other research activities focus on the pathophysiology and therapy of the nephrotic syndrome, biomarker research for acute kidney injury, the role of endothelial progenitor cells and mesenchymal stem cells in various pediatric kidney diseases and the role of the gut microbiome in solid organ transplantation.
Awards and nominations: Eurotransplant Kidney Advisory Committee. Scientific Steering Committee der DZIF Transplantationskohorte e.V.. Chair, Working Group “Renal Transplantation” of the European Society for Paediatric Nephrology (ESPN) and of the German Society for Paediatric Nephrology (GPN). Council of the International Pediatric Transplant Association (IPTA); 2015 - 2017 President of IPTA. Since 2018 Editor-in-Chief of the journal “Pediatric Transplantation”, the official journal of IPTA.
Emulation of the control cohort of a randomized controlled trial in pediatric kidney transplantation with Real-World Data from the CERTAIN Registry
Christian Patry1, Lukas D. Sauer2, Alexander Fichtner1, Luca Dello Strologo3, Dieter Haffner4, Jun Oh5, Ryszard Grenda6, Lars Pape7, Rezan Topaloğlu8, Lutz T Weber9, Antonia Bouts10, Agnieszka Prytula11, Jens König12, Britta Höcker1, Burkhard Tönshoff1.
1Department of Pediatric Nephrology, Zentrum für Kinder -und Jugendmedizin, Heidelberg, Heidelberg, Germany; 2Institute of Medical Biometry, University of Heidelberg, Heidelberg, Germany; 3Renal Transplant Unit, Bambino Gesù Children's Hospital, Pediatric subspecialities, Rome, Italy; 4Department of Pediatric Kidney, Liver and Metabolic Diseases, Hannover Medical School, Hannover, Germany; 5Pediatric Nephrology, University Hospital Hamburg, Hamburg, Germany; 6Department of Nephrology, Kidney Transplantation and Hypertension, Children's Memorial Health Institute, Warsaw, Poland; 7Clinic for Paediatrics III, Essen University Hospital, Essen, Germany; 8Department of Pediatric Nephrology, School of Medicine, Hacettepe University, Ankara, Turkey; 9Pediatric Nephrology, Children’s and Adolescents’ Hospital, University Hospital Cologne, Medical Faculty University of Cologne, Cologne, Germany; 10Department of Pediatric Nephrology, Amsterdam University Medical Center, Emma Children's Hospital, Amsterdam, Amsterdam, Netherlands; 11Pediatric Nephrology and Rheumatology Department, Ghent University Hospital, Ghent, Belgium; 12Department of General Pediatrics, University Children's Hospital, Münster, Münster, Germany
Background: Randomized controlled trials in pediatric kidney transplantation are hampered by low incidence and prevalence of kidney failure in children. Real-World Data from patient registries could facilitate the conduct of clinical trials by substituting a control cohort. However, the emulation of a control cohort by registry data in pediatric kidney transplantation has not been investigated so far.
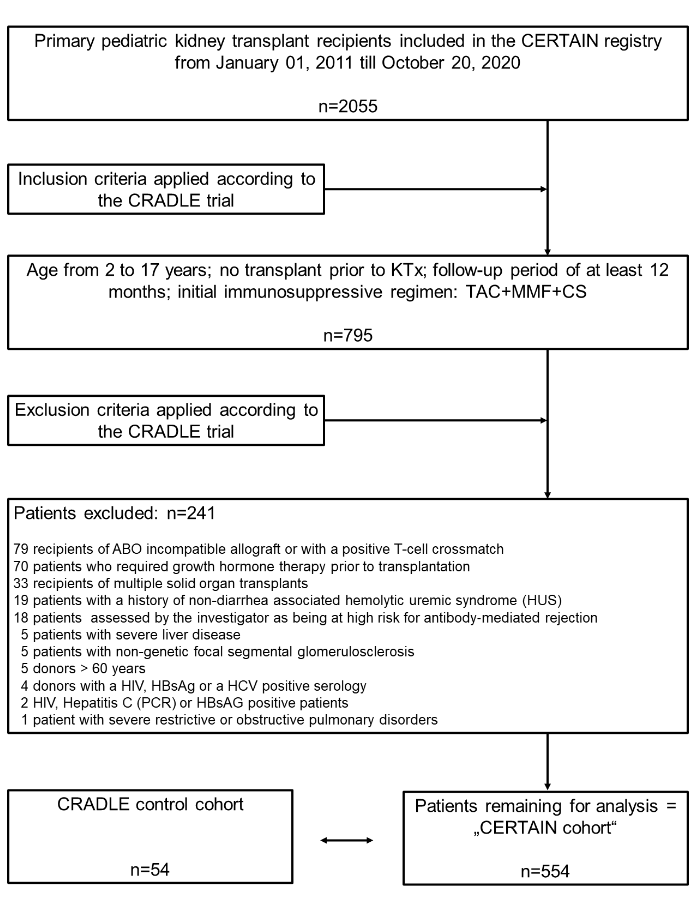
Methods: In this multicenter comparative analysis, we emulated the control cohort (n=54) of an RCT in pediatric kidney transplant patients (CRADLE trial; ClinicalTrials.gov NCT01544491) with data derived from the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN) registry, using the same inclusion and exclusion criteria (CERTAIN cohort, n=554). (Figure 1).
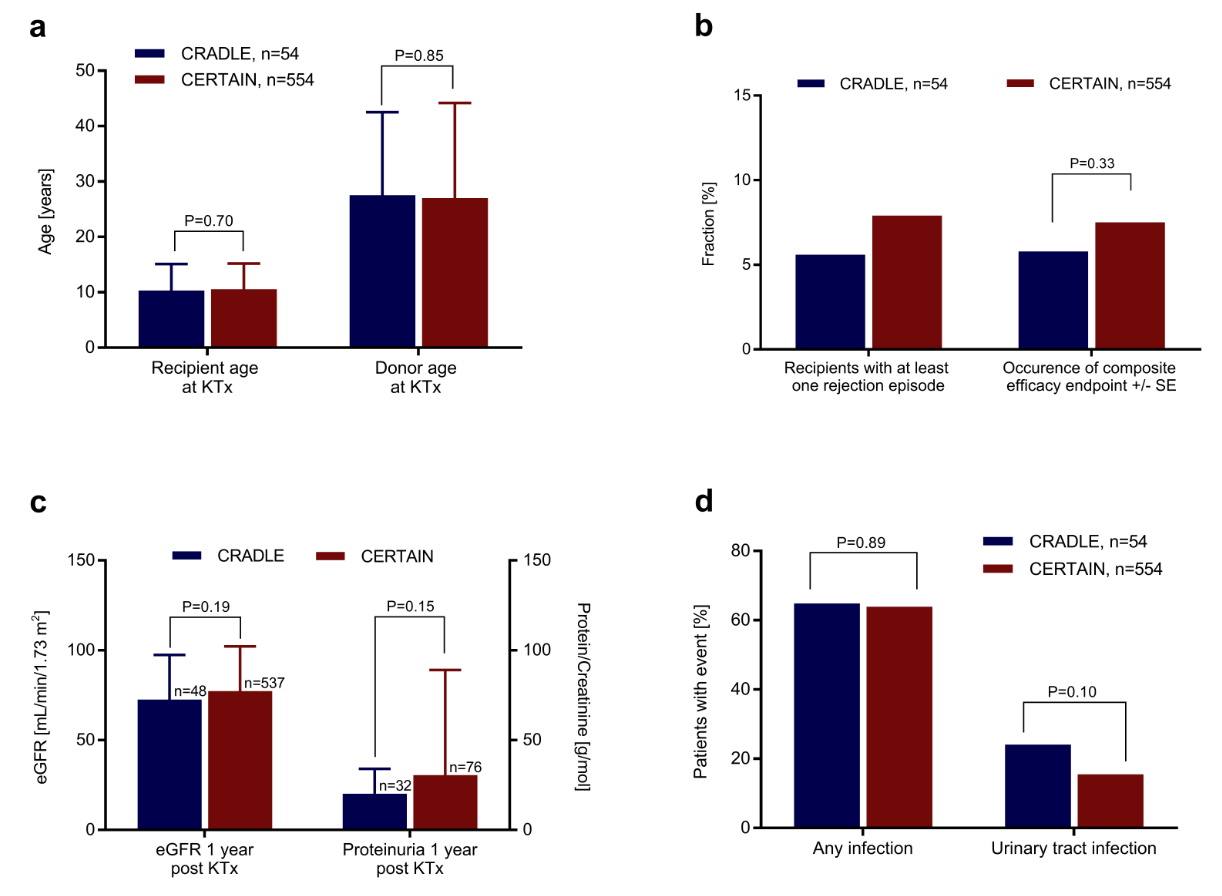
Results: Most baseline patient and transplant characteristics were well comparable between both cohorts. At year 1 posttransplant, a composite efficacy failure end point comprising biopsy-proven acute rejection, graft loss or death (5.8% ± 3.3% vs. 7.5% ± 1.1%, P=0.33) as well as renal function (72.5 ± 24.9 vs. 77.3 ± 24.2 mL/min/1.73 m2 P=0.19) did not differ significantly between CRADLE and CERTAIN. Further, the incidence and severity of BPAR (5.6% vs. 7.8%), the degree of proteinuria (20.2 ± 13.9 vs. 30.6 ± 58.4 g/mol, P=0.15) as well as key safety parameters such as occurrence of urinary tract infections (24.1% vs. 15.5%, P=0.10) were well comparable. (Figure 2).
Conclusions: In conclusion, usage of Real-World Data from patient registries such as CERTAIN to emulate the control cohort of an RCT is feasible and could facilitate the conduct of clinical trials in pediatric kidney transplantation.