Humoral and cellular response to COVID-19 mRNA vaccination in Children with end-stage kidney (ESKD) disease
Carl Grabitz1, Agnes Bonifacius2, Ralf Lichtinghagen3, Gautam Mehta4, Ulrich Baumann1, Britta Eiz-Vesper2, Nele Kanzelmeyer1, Anette Melk1.
1Department of Pediatric Kidney- Liver and Metabolic Diseases, Hannover Medical School, Hannover, Germany; 2Institute of Transfusion Medicine and Transplant Engineering, Hannover Medical School, Hannover, Germany; 3Institute of Clinical Chemistry, Hannover Medical School, Hannover, Germany; 4Institute of Hepatology- Foundation for Liver Research, University College London, London, United Kingdom
Effective mRNA vaccines against SARS-CoV-2 became widespread available during the past year allowing for the unique opportunity to study immune responses to this novel vaccine-type in various naïve populations, both in health and disease. While adult patients with ESKD demonstrated a poor immune response, especially after kidney transplantation (KTx), data for the pediatric population is very limited.
We initiated a longitudinal observational study on humoral and cellular response in children under the age of 16 with ESKD (KTx/dialysis). We measured anti-spike antibodies (against the receptor binding domain) and SARS-CoV-2-specific T cells reactive against spike proteins of several virus variants (ELISPOT assay).
From this still ongoing study, we analyzed 32 children (27 KTx, 5 dialysis) 5-14 weeks after their second dose of BNT162b2/Tozinameran. Mean age of the participants was 12.9±2.72 years, and 13 were under the age of 13. Mean time after KTx or dialysis start was 5.6 years and 1.9 years respectively. Immunosuppression included CNI in combination with mTOR inhibition (n=22) or MMF (n=4), 11 patients additionally received steroids.
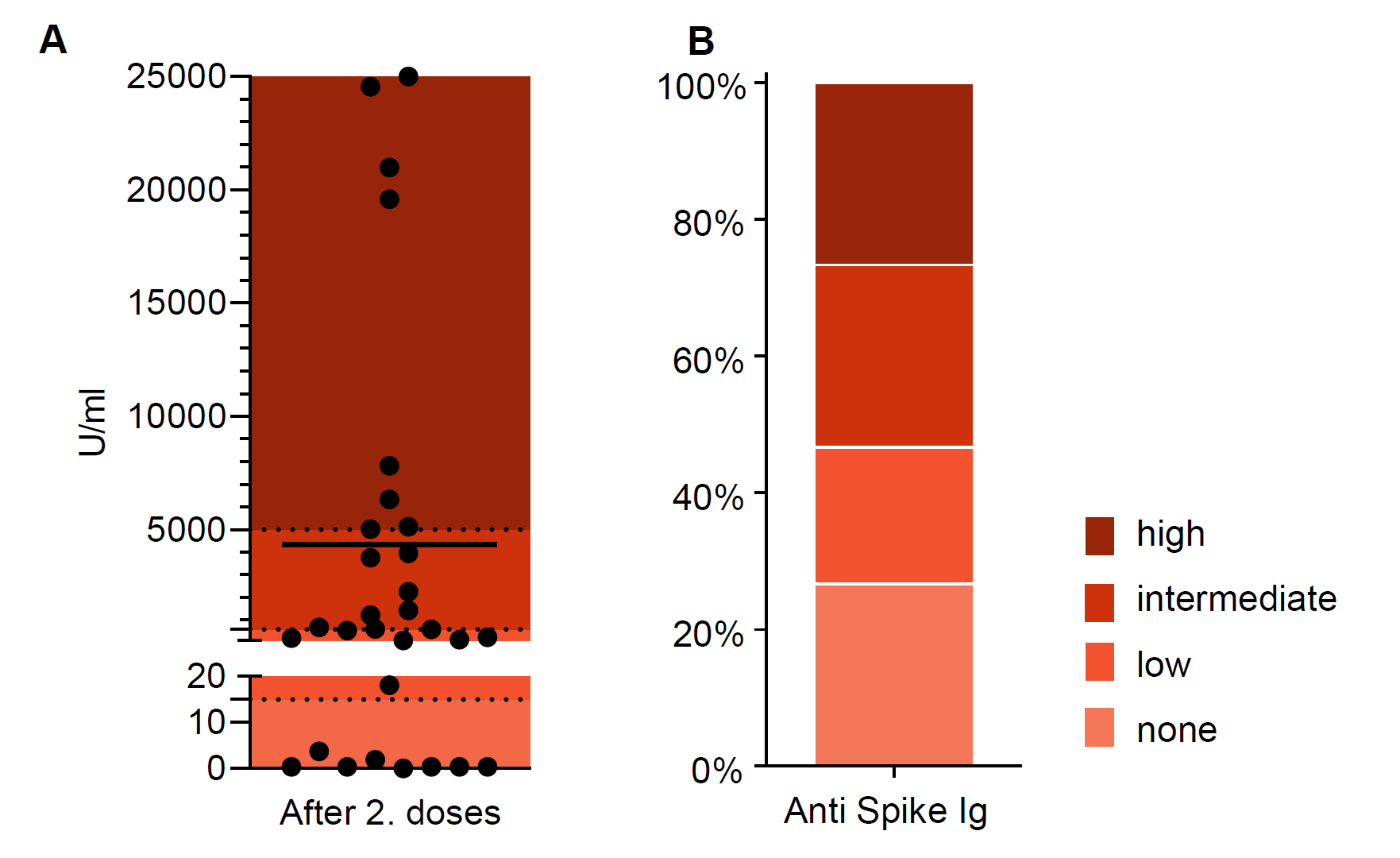
Antibodies were detected in 73%, only 8 patients displayed a strong response (>5,000 U/ml, Fig.1). In contrast, SARS-CoV-2-specific T cells were detected in more than 85% (Fig.2). High and intermediate T cell responses were between 59% and 74% depending on the SARS-CoV-2 variant. Among the total of 8 patients (27%) without detectable antibodies only one individual did not display any T cell response. Younger children under the age of 13 years broadly showed similar immune responses but had less humoral and more cellular non-responders compared with children 13 years and older. The type of immunosuppressive regime did not seem to influence the response to vaccination in this cohort.
In summary, strong immune responses were rare (24% humoral, 19-24% cellular) in our cohort of mainly KTx recipients. More than a quarter did not develop any antibody response, but only about 15% did not show virus-specific T cells. Yet, most of those humoral non-responders had a detectable cellular response. The durability and protection of the observed immune responses will be further followed.
Figure 1:
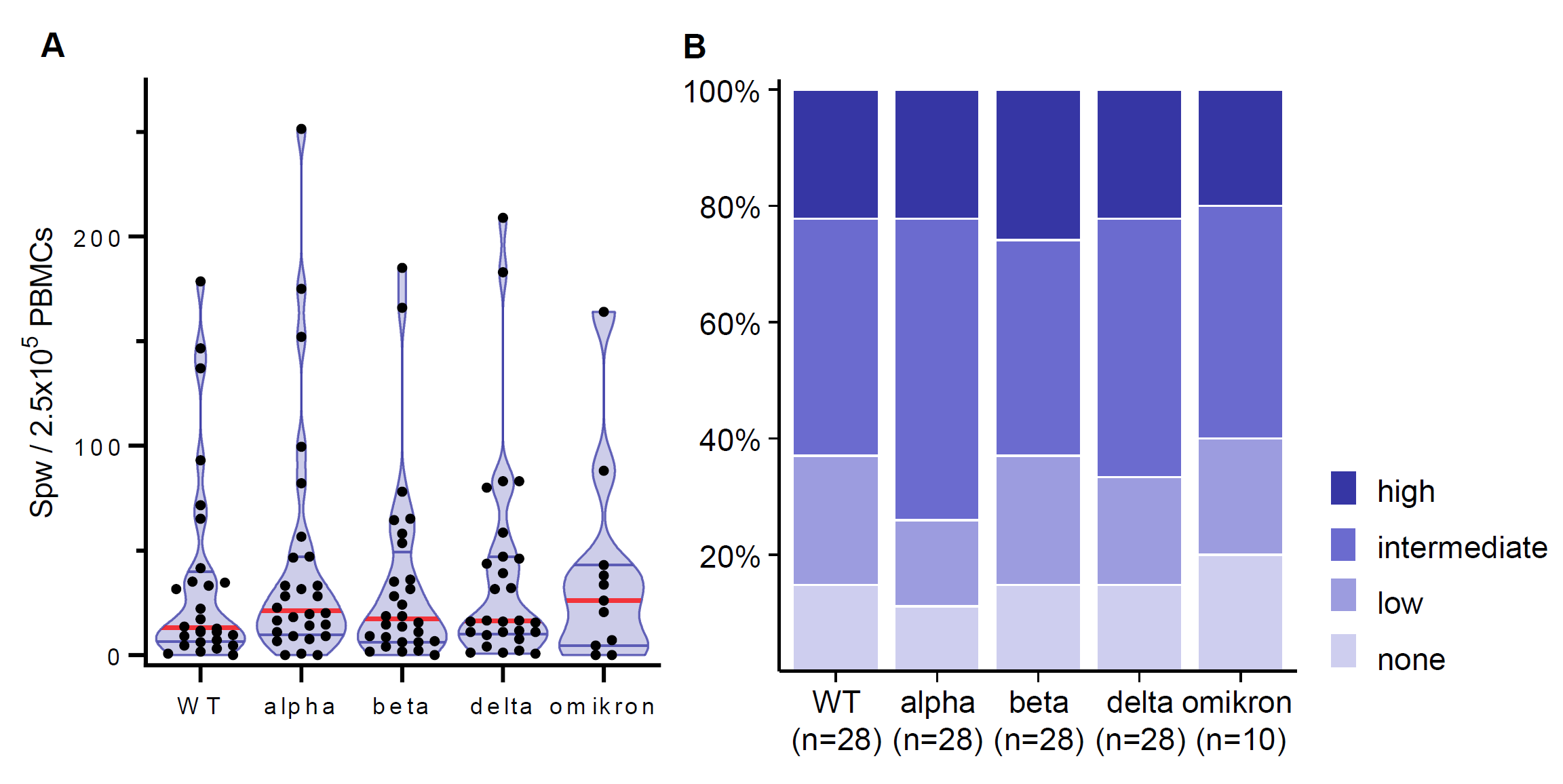
Figure 2: